Preparation method of halogenated alcohol amine
A technology of haloalcoholamine and phenylalanine, which is applied in the field of pharmaceutical intermediates, can solve the problems of difficult synthesis, toxic and harmful reagents, expensive and other problems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0024] The invention provides a preparation method of halohydrin, comprising the following steps:
[0025] L-phenylalanine, benzyl chloroformate, basic compound are mixed with water, carry out amino protection reaction, obtain Cbz-L-phenylalanine;
[0026] The Cbz-L-phenylalanine, thionyl chloride and methanol are mixed, and esterification is carried out to obtain Cbz-L-phenylalanine methyl ester;
[0027] Mixing the Cbz-L-phenylalanine methyl ester, diisobutylaluminum hydride and toluene, and carrying out a reduction reaction to obtain Cbz-L-phenylalanine aldehyde;
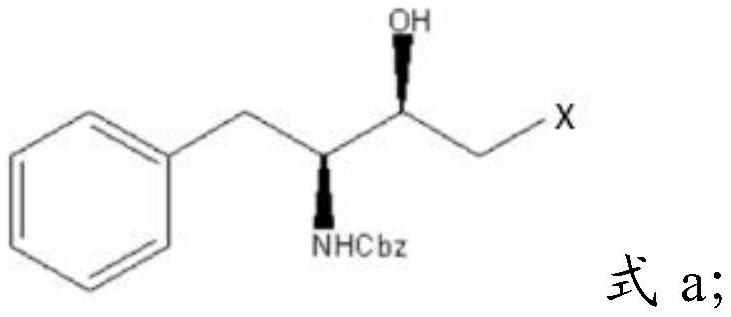
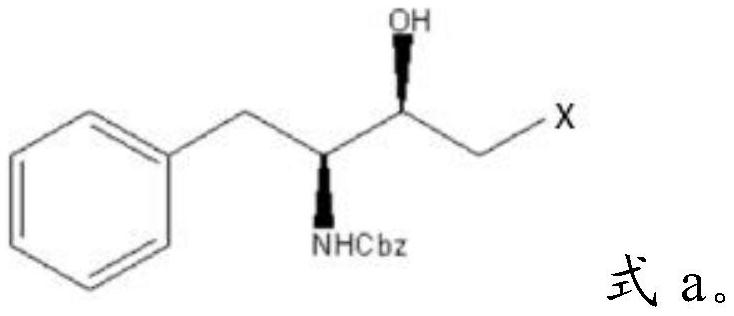
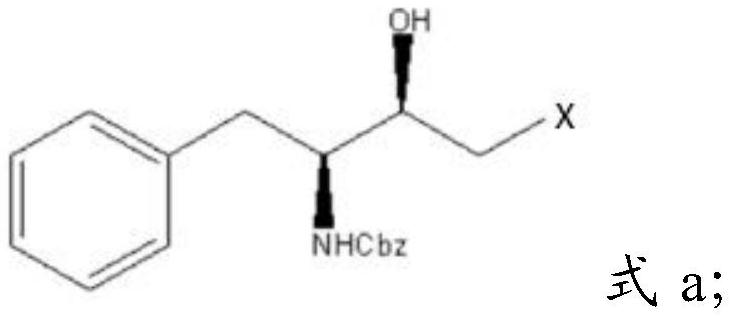
[0028] Mixing described Cbz-L-phenylalaninaldehyde, dihalomethane, isopropylmagnesium chloride and tetrahydrofuran, carrying out substitution reaction to obtain halohydrin having the structure shown in formula a;
[0029]
[0030] In formula a, X is a halogen group.
[0031] In the present invention, L-phenylalanine, benzyl chloroformate, basic compound and water are mixed to carry out amino protection react...
Embodiment 1
[0044] The preparation of Cbz-L-phenylalanine includes the following steps:
[0045] The L-phenylalanine (16.5g, 0.1mol) solid was added to 400mL of water, stirred to dissolve, and Na 2 CO 3 (15.9g, 0.15mol), cool down to 0~8℃, stir to dissolve, add benzyl chloroformate (17.9g, 0.105mol) dropwise to the obtained system, control the temperature to maintain at 0~8℃, under stirring conditions The amino protection reaction was carried out for 3 h; after the reaction, the pH value of the obtained product system was adjusted to 2 with hydrochloric acid, extracted with ethyl acetate (500 mL × 3 times), and the obtained organic phase was washed with saturated brine (500 mL × 1 time), After drying over anhydrous sodium sulfate, the obtained filtrate was filtered and concentrated to obtain Cbz-L-phenylalanine with a yield of 26.9 g, a yield of 90%, and a purity of 99.0%.
Embodiment 2
[0047] The preparation of Cbz-L-phenylalanine aldehyde comprises the following steps:
[0048] Cbz-L-phenylalanine (24 g, 80.2 mmol) was stirred and mixed with 200 mL of anhydrous methanol, thionyl chloride (23.8 g, 0.2 mol) was added, and the obtained system was heated to reflux for 3 h; after the reaction was completed, The obtained product system was concentrated to dryness, 500 mL of dichloromethane was added to dissolve the obtained residue, then washed with saturated sodium bicarbonate solution and saturated brine in sequence, dried over anhydrous sodium sulfate, filtered and concentrated to the obtained filtrate to obtain the crude product, The crude product was distilled under high vacuum (2-3 mmHg) to obtain a pale yellow ester product, namely Cbz-L-phenylalanine methyl ester, the yield was 21.4 g, the yield was 85%, and the purity was 99.1% .
[0049] Cbz-L-phenylalanine methyl ester (20g, 63.8mmol) was dissolved in 200mL of toluene, then under nitrogen protection, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com