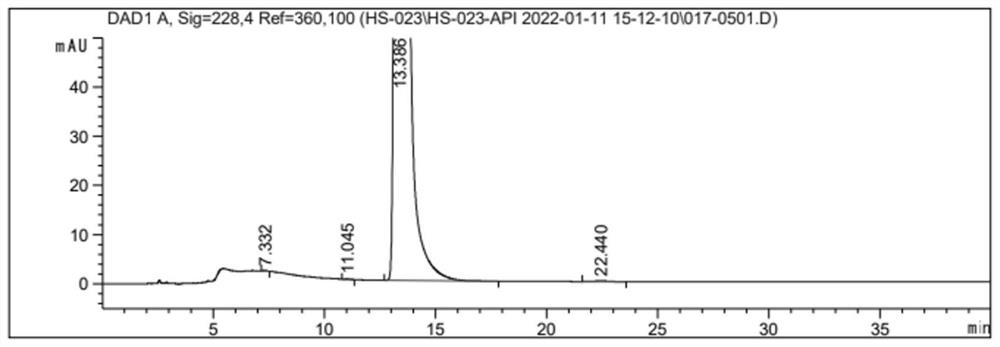
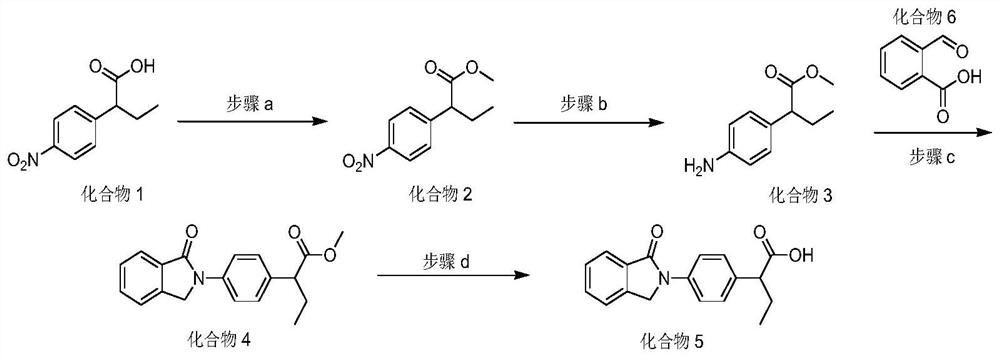
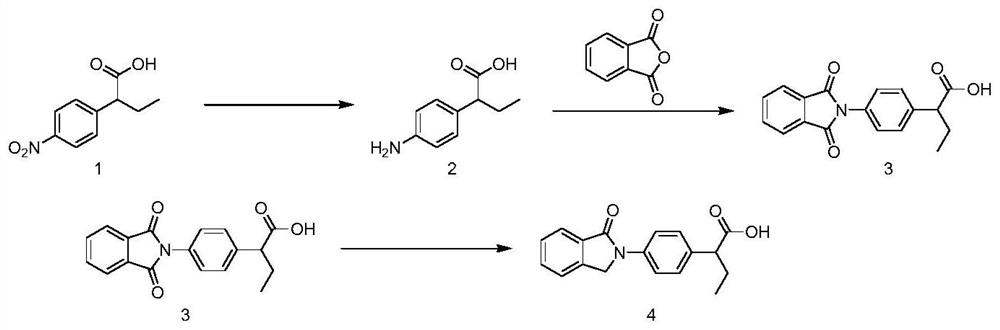
Method for preparing indobufen
A technology of indobufen and its compounds, which is applied in the field of preparation of indobufen, can solve the problems of low product purity and difficult removal, and achieve the effects of simple purification process, material cost saving, and simple post-treatment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] 1. Synthesis of compound 2
[0037] Dissolve 2-(4-nitrophenyl)butyric acid (50g, 1.0eq) in methanol, add concentrated sulfuric acid, heat under reflux for 2h with stirring, TLC detects the end of the reaction, and the reaction solution can be directly put into the next step.
[0038] 2. Synthesis of compound 3
[0039] To the reaction solution in the previous step, add reduced iron powder (46.4g, 3.5eq), add concentrated hydrochloric acid dropwise at room temperature, react for 3 hours, remove the solvent after TLC detection, add water and ethyl acetate, and add solid sodium carbonate The pH was adjusted to about 8, the layers were separated, the aqueous layer was extracted twice with ethyl acetate, the organic phases were combined, dried, filtered, and the filtrate was concentrated to obtain compound 3 with a yield of 85.6%.
[0040] 3. Synthesis of compound 4
[0041] Compound 3 (20g, 1.0eq), o-carboxybenzaldehyde (17.1g, 1.1eq), and sodium borohydride (1.96g, 0.5eq...
Embodiment 2
[0047] 1. Synthesis of compound 2
[0048] Dissolve 2-(4-nitrophenyl)butyric acid (50g, 1.0eq) in methanol, add concentrated sulfuric acid, heat under reflux for 2h with stirring, TLC detects the end of the reaction, and the reaction solution can be directly put into the next step.
[0049] 2. Synthesis of compound 3
[0050] To the reaction solution in the previous step, add reduced iron powder (60.1g, 4.5eq), add concentrated hydrochloric acid dropwise at room temperature, react for 3 hours, remove the solvent after TLC detection, add water and ethyl acetate, and add sodium carbonate solid The pH was adjusted to about 8, the layers were separated, the aqueous layer was extracted twice with ethyl acetate, the organic phases were combined, dried, filtered, and the filtrate was concentrated to obtain compound 3 with a yield of 85.3%.
[0051] 3. Synthesis of compound 4
[0052] Compound 3 (20g, 1.0eq), o-carboxybenzaldehyde (17.1g, 1.1eq), formic acid (7.15g, 1.5eq), triethylam...
Embodiment 3
[0057] 1. Synthesis of compound 2
[0058] Dissolve 2-(4-nitrophenyl)butyric acid (50 g, 1.0 eq) in methanol, add concentrated sulfuric acid, heat under reflux for 2 h with stirring, TLC detects the end of the reaction, and the reaction solution can be directly put into the next step.
[0059] 2. Synthesis of compound 3
[0060] Zinc powder (62.5g, 4eq) was added to the reaction solution in the previous step, concentrated hydrochloric acid was added dropwise at room temperature, reacted for 3 hours, the solvent was removed after the reaction was detected by TLC, water and ethyl acetate were added, and sodium carbonate solid was added to adjust the pH. Adjusted to about 8, separated, the aqueous layer was extracted twice with ethyl acetate, the organic phases were combined, dried, filtered, and the filtrate was concentrated to obtain compound 3 with a yield of 85.6%.
[0061] 3. Synthesis of compound 4
[0062] Compound 3 (20g, 1.0eq), o-carboxybenzaldehyde (17.1g, 1.1eq), an...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com