Doxercalciferol and preparation method thereof
A technology for the chemical reaction of doxercalcidol, applied in the field of doxocalcidol and its preparation, can solve the problems of difficult to control product purity, difficult to standardize operation, unstable yield, etc., and achieve great application value and economic value, Mild reaction and good product purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
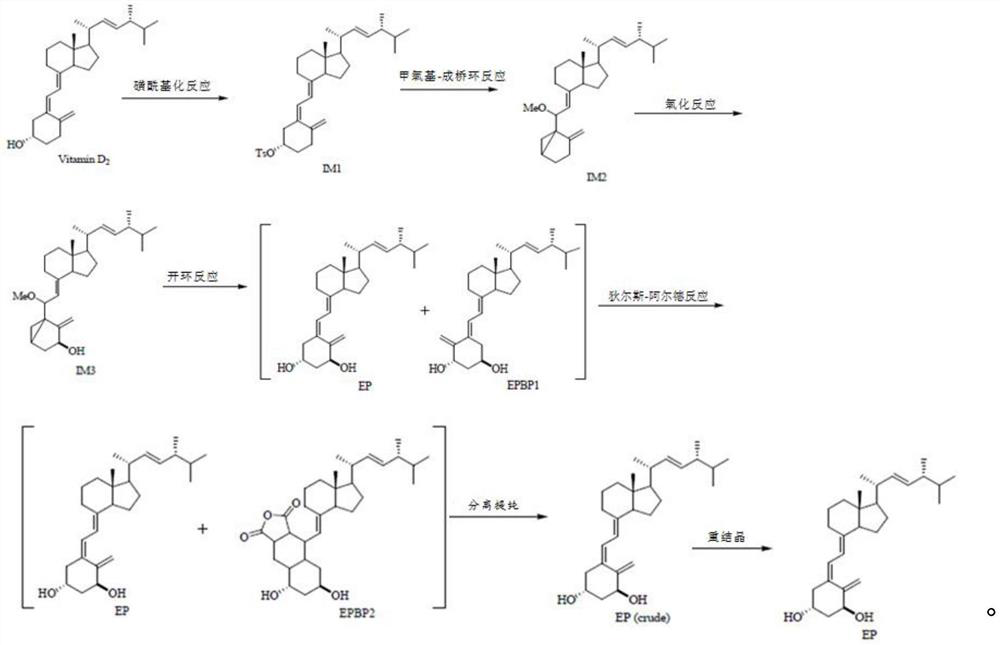
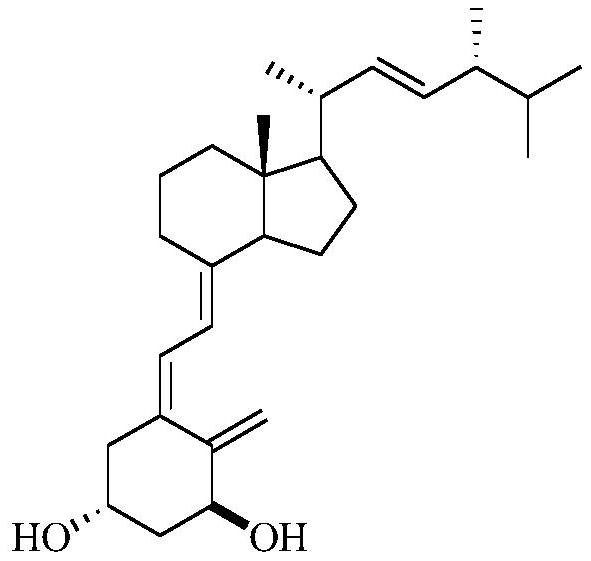
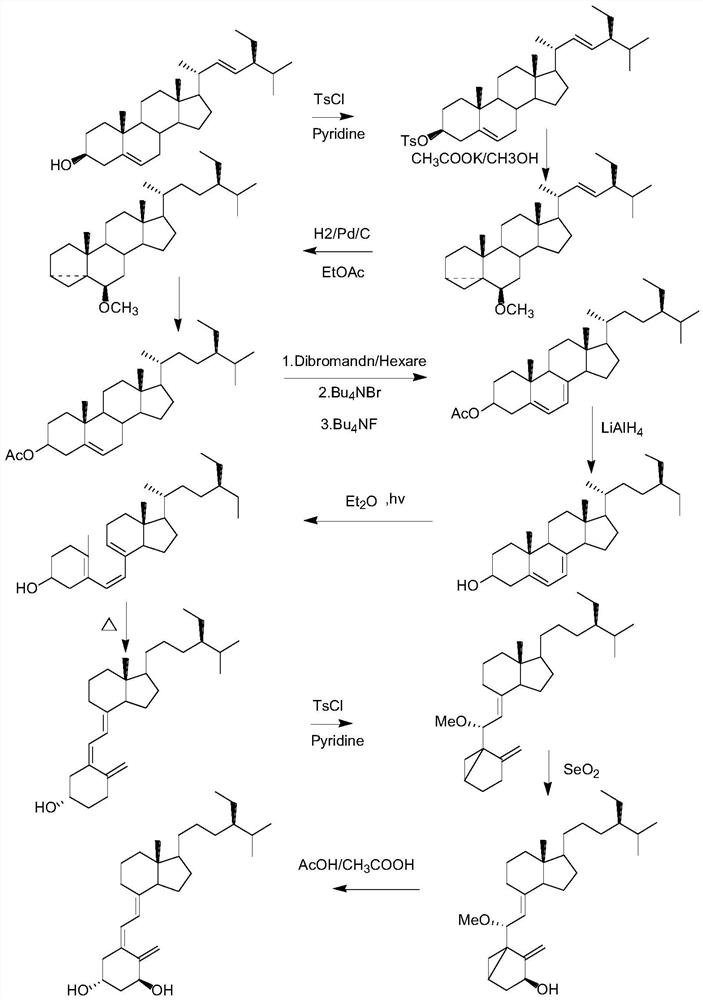
[0050] The synthetic reaction chemical equation of the preparation method of described calciferol is:
[0051]
Embodiment 1
[0053] A preparation method of calciferol, comprising the following steps:
[0054] S1 Vitamin D 2 Synthesis of intermediate vitamin D by sulfonylation reaction 2 p-toluenesulfonate ester (IM1), comprising the following steps:
[0055] Under the protection of nitrogen, add 4.0kg of vitamin D to the reaction kettle in sequence 2 , 14L of dichloromethane, 4kg of pyridine, stirred until dissolved, cooled the reaction system to 0°C, added 0.2kg of 4-dimethylaminopyridine and 4.0kg of p-toluenesulfonyl chloride, reacted at 0°C for 71h, and controlled the temperature to 0 ℃, slowly add 10L deionized water to the reaction solution to quench the reaction, after the addition, continue to stir for 10min, let stand to separate the liquid, separate the organic phase, adjust the pH to 1 with 1M sulfuric acid, stand to separate, and use the organic layer The saturated sodium carbonate solution was adjusted to pH = 7, the organic layer was separated, stirred and dried with 2.5 kg of anhyd...
Embodiment 2
[0081] A preparation method of calciferol, comprising the following steps:
[0082] S1 Vitamin D 2 Synthesis of intermediate vitamin D by sulfonylation reaction 2 p-toluenesulfonate ester (IM1), comprising the following steps:
[0083] Under nitrogen protection conditions, add dichloromethane, 5.0kg vitamin D to the reactor 2 , pyridine, stirred until dissolved, cooled the reaction system to 5°C, added 4-dimethylaminopyridine and p-toluenesulfonyl chloride, reacted at 5°C for 48 hours, controlled the temperature at 5°C, and added deionized water dropwise after the reaction was complete Quenching the reaction, adding and continuing to stir to obtain a mixed solution, the mixed solution was left to separate liquids, and the organic phase was separated, adjusted to pH=1 with sulfuric acid, left to separate layers, and the organic layer was adjusted to pH=8 with saturated sodium bicarbonate solution, Separate the organic layer and stir and dry it with anhydrous magnesium sulfat...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com