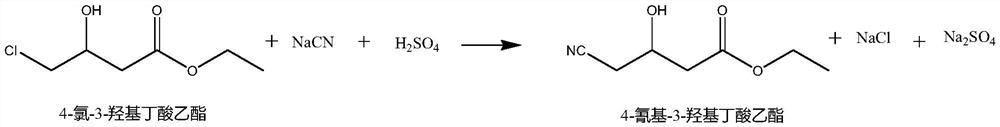
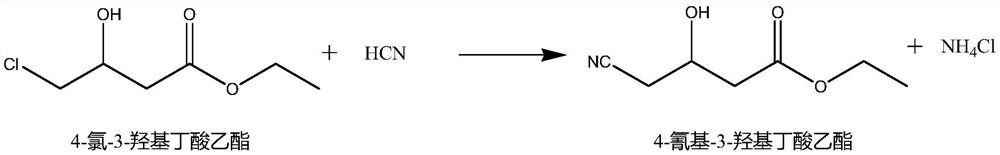
Synthesis method of ethyl 4-cyano-3-hydroxybutyrate
A technology of ethyl hydroxybutyrate and a synthesis method, which is applied in the direction of carboxylic acid nitrile purification/separation, organic chemistry, fermentation, etc., can solve the problems of large amount of extractant, large amount of sodium cyanide, and high operation cost, and achieves an increase in the The effect of technological advancement, environmental protection cost reduction, and mild reaction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] A kind of 4-cyano group-3-hydroxybutyrate ethyl ester synthetic method, comprises the following steps:
[0037] (1) Reaction synthesis: add 80ml of deionized water in the reaction flask, drop into 17.3g (0.1mol) of 4-chloro-3-hydroxybutyrate ethyl ester (purity 96%), add ammoniacal liquor (25%) to adjust pH6. 9-7.1, add 0.85g dehalogenase, then pass through hydrocyanic acid, and add ammonia water (25%) solution to adjust the pH to 6.9-7.1, control the temperature at 40-55°C, and react until the analysis of 4-chloro-3- The ethyl hydroxybutyrate content is ≤1%, and then spot the plate again to confirm that the reaction is complete, confirm the end point of the reaction, and obtain the reaction liquid;
[0038] (2) Precipitation and water removal: the reaction solution is concentrated and dehydrated, the temperature is controlled below 60°C, the vacuum is at 15Kpa, and concentrated to 35-45% of the volume of the feed liquid to obtain the feed liquid;
[0039] (3) Desaltin...
Embodiment 2
[0043] A kind of 4-cyano group-3-hydroxybutyrate ethyl ester synthetic method, comprises the following steps:
[0044] (1) Reaction synthesis: add 80ml of deionized water into the reaction flask, drop into 17.3g (0.1mol) of ethyl 4-chloro-3-hydroxybutyrate (purity 96%), add methylamine water (40%) solution Adjust the pH to 6.9-7.1, add 0.85g of dehalogenase, then pass through hydrocyanic acid, add methylammonia (40%) solution at the same time to adjust the pH to 6.9-7.1, control the temperature at 40-55°C, and react until the analysis of 4- The content of ethyl chloro-3-hydroxybutyrate is ≤1%, and then spot the plate again to confirm that the reaction is complete, confirm the end point of the reaction, and obtain the reaction solution;
[0045] (2) Precipitation and water removal: the reaction solution is concentrated and dehydrated, the temperature is controlled below 60°C, the vacuum is at 15Kpa, and each batch is concentrated to 35-45% of the volume of the feed liquid to ob...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com