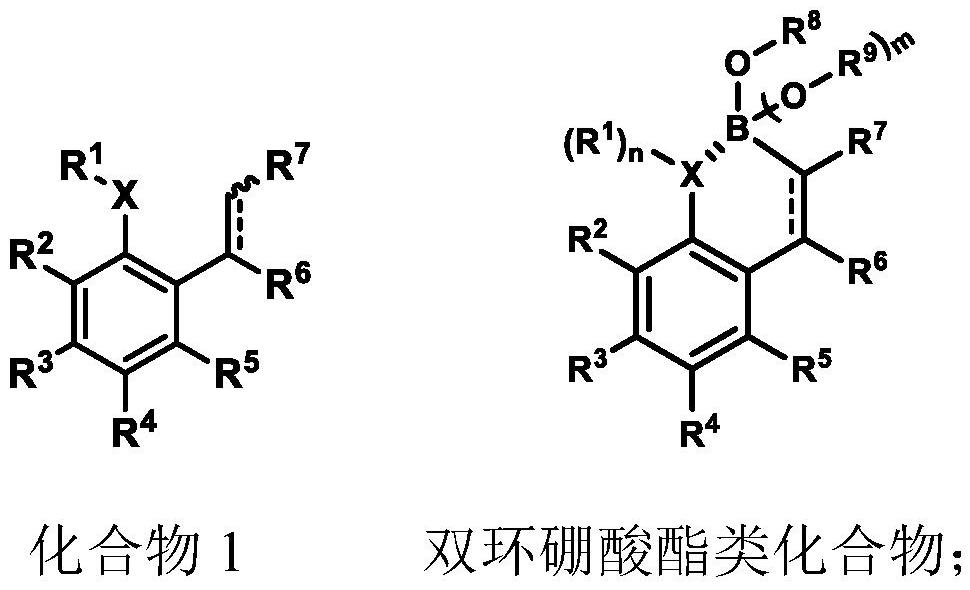
Preparation method and application of bicyclic borate compound
A technology of bicyclic boronic acid esters and compounds, which is applied in the field of preparation of bicyclic boronic acid ester compounds, can solve problems affecting research development, etc., and achieve the effects of improving synthesis yield, shortening synthesis steps, and simple preparation methods
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-19
[0062] Embodiment 1-19 provides a kind of bicyclic boronate ester compound respectively, and its general preparation method is as follows:
[0063]
[0064] 1.0 mL of DCM was added to a dry nitrogen-protected reaction tube, and after cooling at -60 °C, the BBr 3 (0.22 mmol, 1.1 equiv; 1.0 mol / L in DCM), base (0.22 mmol, 1.1 equiv, 2,6-di-tert-butylpyridine in Example 1-18, 2,3 in Example 19, 5,6-Tetramethylpyrazine) was dissolved and added dropwise to the solution. After 5 minutes, compound 1 (0.20 mmol, 1.0 equiv) was added dropwise, and the mixture was stirred at -60° C. for another 2.0 hours (in Example 19, it was at 0 °C stirring for 1.5h). Finally, the resulting reaction mixture was washed with base (1.0 equiv), methanol (1.0 mL) and H 2 O (0.2 mL) quenched. Most of the solvent was then spun off under reduced pressure and the crude product was purified by silica gel chromatography (PE / EA (5:1)) to give the desired product Compound A.
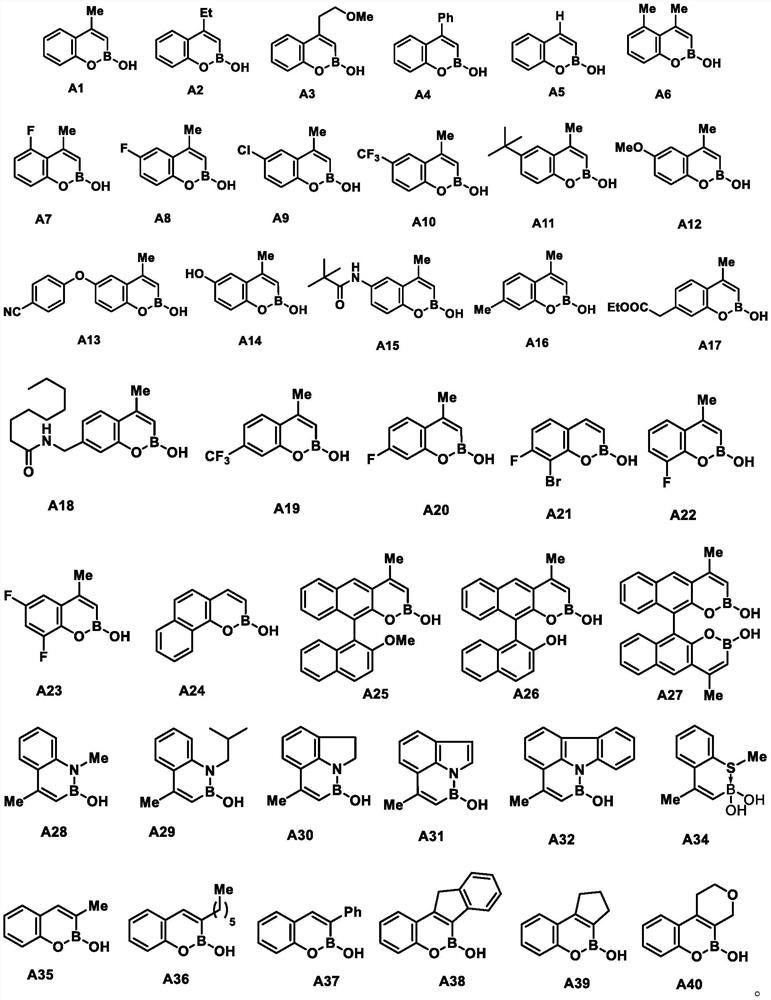
[0065] Substitute different s...
Embodiment 1
[0102]
[0103] Compound A1, white solid, 30 mg, 95% yield; mp 136-138°C; 1 H NMR (600MHz, MeOD) δ 7.63(d, J=7.9Hz, 1H), 7.38(s, 1H), 7.28–7.23(m, 1H), 7.17(s, 1H), 5.98(s, 1H) ,2.41(d,J=1.1Hz,3H); 13 C NMR (151MHz, MeOD) δ155.46, 152.49, 128.95, 124.95, 124.92, 121.88, 118.24, 20.84; 11 B NMR(193MHz,MeOD)δ27.02.HRMS(ESI): Calculated 159.0727, the measured value is 159.0726.
Embodiment 2
[0105]
[0106] Compound A2, white solid, 31 mg, 89% yield; melting point 150-152°C; 1 H NMR (600MHz, MeOD) δ 7.68 (d, J=6.6Hz, 1H), 7.39–7.35 (t, 1H), 7.27 (d, J=7.1Hz, 1H), 7.17 (t, J=7.0Hz) ,1H),6.00(s,1H),2.79(q,J=7.4,1.0Hz,2H),1.28(t,J=7.4Hz,3H); 13 C NMR (151MHz, MeOD) δ160.87, 152.72, 128.82, 124.48, 124.15, 121.84, 118.50, 26.80, 11.93; 11 B NMR (193 MHz, MeOD) δ 27.22.; HRMS (ESI): Calculated 213.1246, found 213.1242.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com