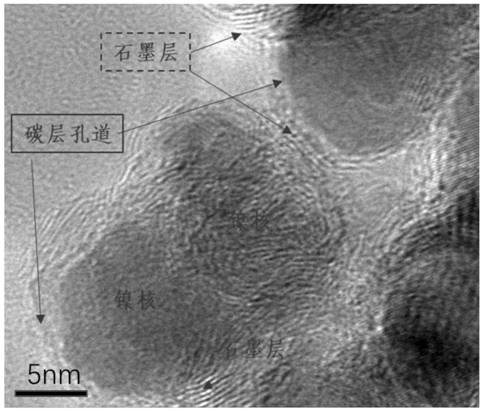
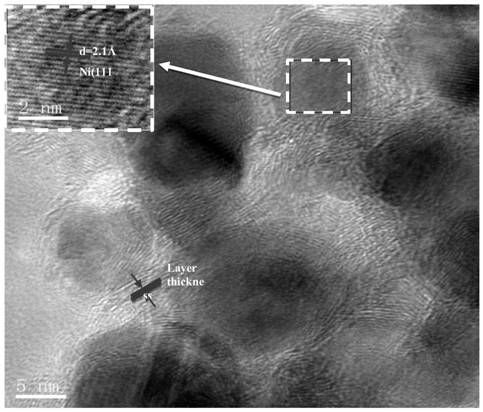
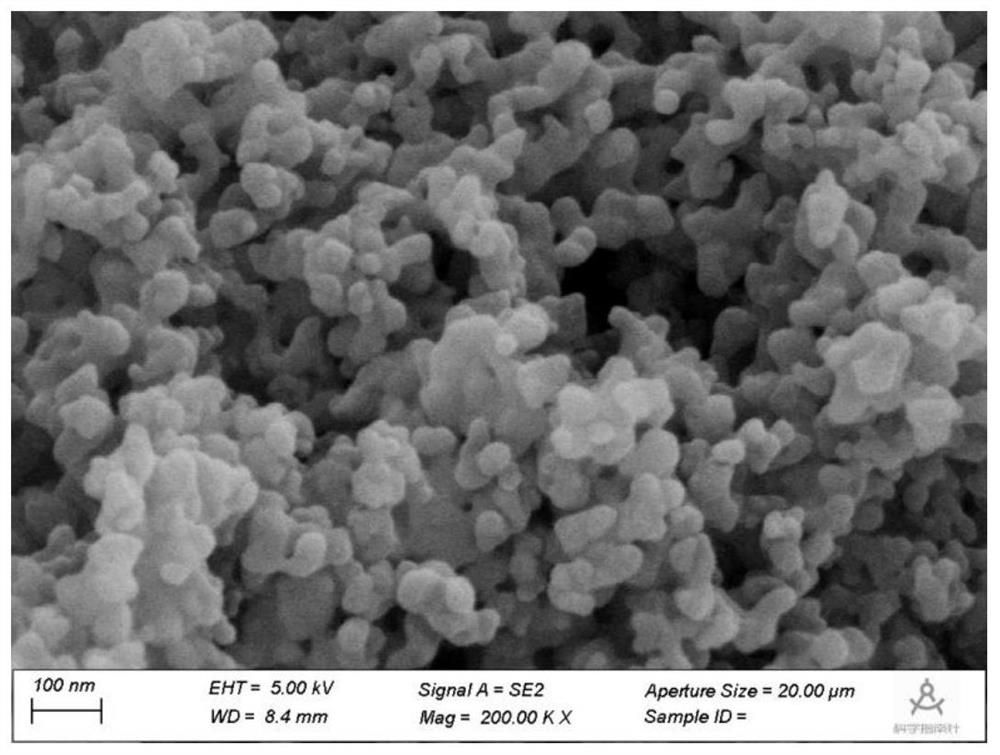
Application of carbon material coated nickel nanoparticle catalyst in synthesis of p-aminophenylacetic acid by hydrogenation of p-nitrophenylacetic acid
A technology of p-nitrophenylacetic acid and p-aminophenylacetic acid is applied in the preparation of organic compounds, physical/chemical process catalysts, metal/metal oxide/metal hydroxide catalysts, etc. "Three wastes" treatment of large investment, catalyst can not be reused and other problems, to achieve the effect of green and efficient preparation method, low production cost, easy to operate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0076] Weigh a certain amount of nickel nitrate hexahydrate and 4-pyridinecarboxylic acid and dissolve in a certain amount of absolute ethanol, the molar ratio of nickel to organic ligand is 1:2; the mass ratio of nickel to alcohol solution is 1:100. Heat at 25°C After stirring for 6 hours, add 1.0 mol / L sodium hydroxide aqueous solution dropwise to the mixed solution, adjust the pH of the solution to 4.0, and keep for 1 hour; add dropwise 98% triethanolamine solution, adjust the pH to 6.5, and keep for 2 hours; Add 25-28% ammonia water dropwise, adjust the pH to 8.5, and keep it for 2 hours. The resulting slurry is sealed and placed on a vibration table. The vibration program is set as follows: 1) Vibration frequency 10Hz, banner vibration for 2 minutes; 2) Vibration frequency 30Hz, banner vibration for 5 minutes; 3) Vibration frequency 40Hz, banner vibration for 1 minute; 4) The operation procedure 1)-3) is a vibration cycle; the entire crystallization, precipitation and agi...
Embodiment 16
[0118] Example 16 investigated the performance of different nickel nanoparticle catalysts prepared in Examples 1-15 and Comparative Examples 1-16 in catalytic hydrogenation synthesis of haloanilines.
[0119] In a 50ml stainless steel reaction kettle, add 25ml methanol, 1.0g p-chloronitrobenzene, 0.05g carbon material coated nickel nanoparticle catalyst prepared by different embodiments or comparative examples, close the reaction kettle, and replace the air in the reaction kettle with hydrogen for 10 The second time, the temperature was raised to 100°C, the hydrogen pressure was 1.0 MPa, stirring was started, and the stirring rate was 1800 r / min, and the reaction was carried out for 40 minutes. Stop the reaction, and after the temperature drops to room temperature, take the supernatant of the reaction solution, filter the catalyst, and analyze the filtrate by gas chromatography. The experimental results are shown in Table 3:
[0120] Table 3 Performance of different carbon ma...
Embodiment 17
[0124] Example 17 investigated the reaction performance of the carbon material-coated nickel nanoparticle catalyst prepared in Example 2 for the hydrogenation of different halogenated nitrobenzenes to prepare halogenated anilines.
[0125] In a 50ml stainless steel reactor, add 25ml of methanol, 1.0g of different halogenated nitrobenzenes, 0.05g of the carbon material coated nickel nanoparticle catalyst prepared in Example 2, close the reactor, and replace the air in the reactor with hydrogen for 10 times , the temperature was raised to 100° C., the hydrogen pressure was 1.0 MPa, stirring was started at a stirring rate of 1800 r / min, and the reaction was carried out for 1 h. Stop the reaction, and after the temperature drops to room temperature, take the supernatant of the reaction solution, filter the catalyst, and analyze the filtrate by gas chromatography. The experimental results are shown in Table 4:
[0126] Table 4 Reaction performance of nickel nanoparticle catalysts ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com