Fusion protein and application thereof in preparation of tumor drugs
A fusion protein and amino acid technology, applied in the field of tumors, can solve the problems that hinder the clinical application of IL-2, short half-life, toxic and side effects, etc., and achieve the effect of having immune memory, weak toxic and side effects, and preventing recurrence and metastasis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
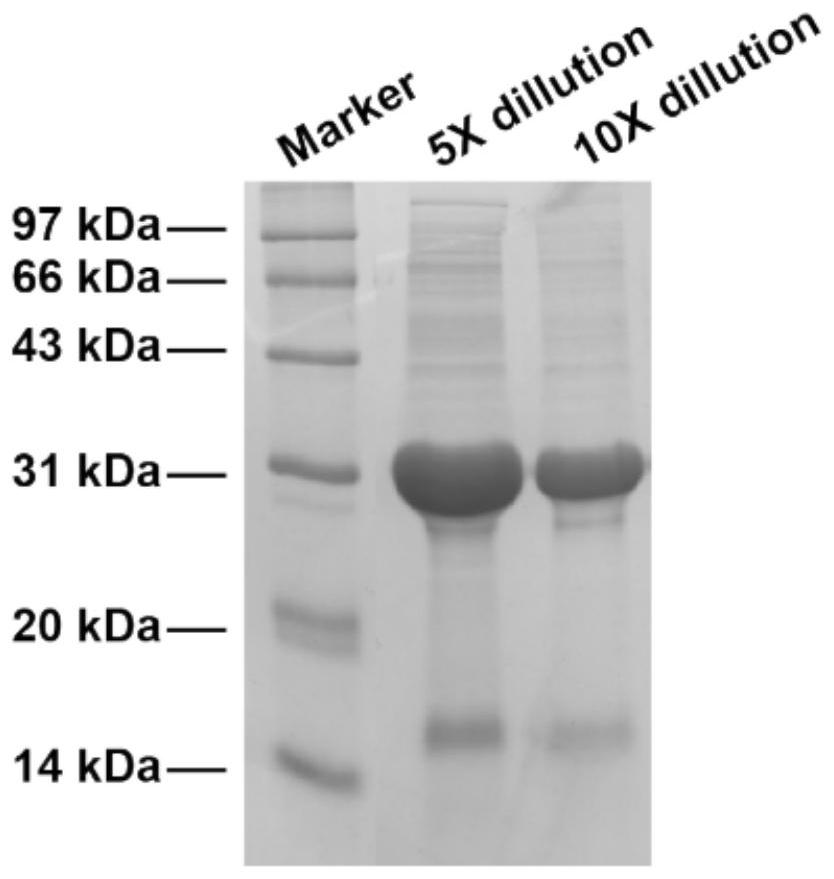
[0057] Example 1 pHLIP-IL2 de novo synthesis (de novo synthesis) and purification
[0058] 1. Synthesis of pHLIP-IL2 de novo and construction of expression vector
[0059] The C-terminus of the IL-2 amino acid sequence (shown in SEQ ID NO: 1) was linked to the N-terminus of the pHLIP amino acid sequence (shown in SEQ ID NO: 2) by a 2 amino acid peptide (GS). The corresponding cDNA was synthesized according to the designed amino acid sequence (as shown in SEQ ID NO: 3), cloned into the pET30a plasmid between the EcoRI site and the XhoI site by PCR, and the expression vector was constructed and verified successfully to obtain the expression vector.
[0060] 2. Construction of recombinant strains
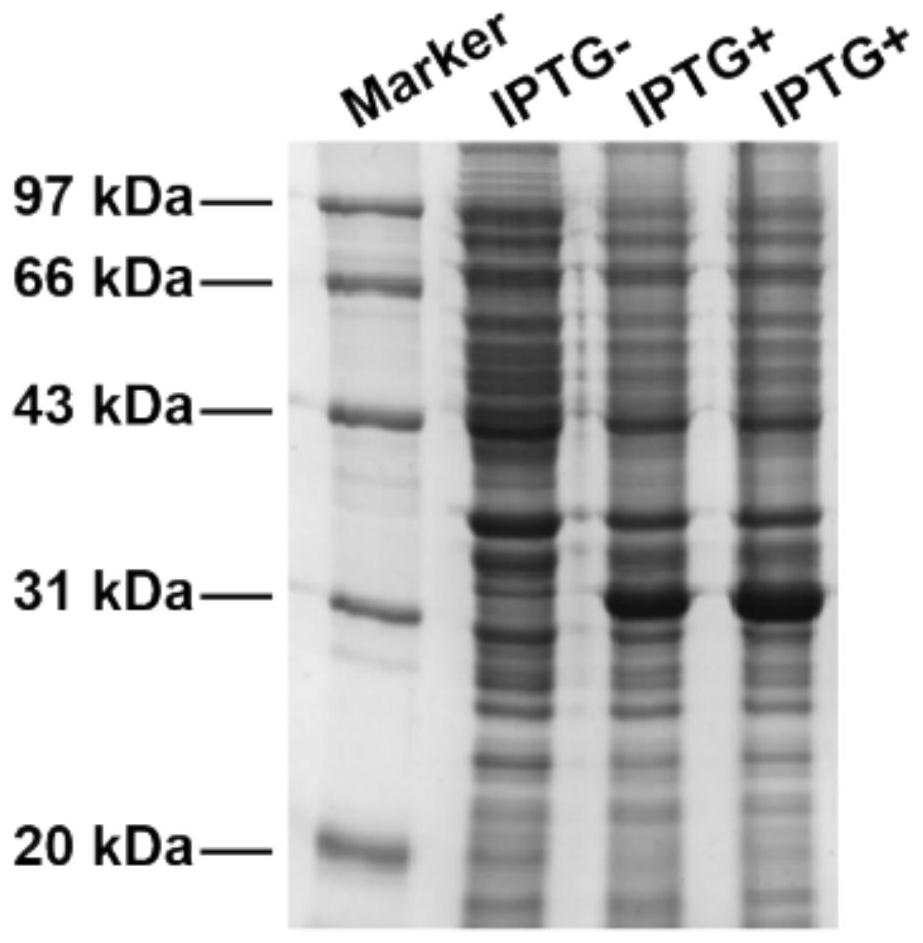
[0061] Transform the constructed expression vector into the expression strain. Take out the BL21 competent bacteria stored at -80°C and thaw slowly on ice, add the previously synthesized expression vector, and place on ice for 30 minutes. Subsequently, the competent cells were heat-...
Embodiment 2
[0080] Example 2 pHLIP-IL2 chemical coupling synthesis
[0081] 1. Experimental method
[0082] Sulfo-SMCC was used as the linker. Sulfo-SMCC has both a Sulfo-NHS ester group and a maleimide group as a cross-linking agent. Under the condition of pH 7~9, the NHS group can react with the primary amine group of the protein to form an amide bond. Under the condition of pH 6.5~7.5, the maleimide group can react with the sulfhydryl group of the protein to form a thioether bond.
[0083] In order to achieve the purpose of covalent coupling, the pHLIP polypeptide sequence was designed, and a cysteine was added to the N-terminus of the pHLIP original sequence (as shown in SEQ ID NO: 2) for subsequent connection with the maleimide group. reaction. The redesigned synthetic peptide was synthesized by solid-phase peptide synthesis, and the purity was verified by HPLC and the molecular weight was verified by MS.
[0084] Dissolve 1 mg of IL-2 lyophilized powder (as shown in SEQ ID NO:...
Embodiment 3
[0087] Example 3 Detection of pHLIP-IL2 acidic pH response characteristics by flow cytometry
[0088] 1. Experimental method
[0089] 1. Fluorescently label the pHLIP-IL2 fusion protein.
[0090] Dip a 20-fold molar excess of fluorescent dye Cy5.5-NHS into 100 μl DMSO with a 1 ml pipette tip, dissolve it in 100 μl DMSO, add 900 μl PBS Buffer, dilute it by pipetting evenly, take 500 μl and add 1 ml of 0.5 mg / ml pHLIP- IL2 fusion protein solution, react at 4°C for 4 hours. Then, the excess Cy5.5-NHS in the protein was removed by ultrafiltration with a 3kD ultrafiltration tube at 12000rpm for 30 minutes, and the Cy5.5-labeled pHLIP-IL2 fusion protein was obtained.
[0091] 2. The pHLIP-IL2 fusion protein reacts with cells
[0092] One tube of B16 cells was recovered, cultured in 10% FBSDMEM medium, and plated in six-well plates one day in advance after passage and expansion, and 105 cells were plated in each well. After one day of culture, the original medium was removed, was...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com