High-activity blood coagulation factor VIII or VIII polypeptide variant Gly710Thr
A blood coagulation factor and highly active technology, applied in the field of hemophilia, can solve the problems of failure of replacement therapy, heavy economic burden, long-term preventive treatment of heavy economic burden, etc., achieve superior stability, improve drug efficacy, and good clinical application prospects Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
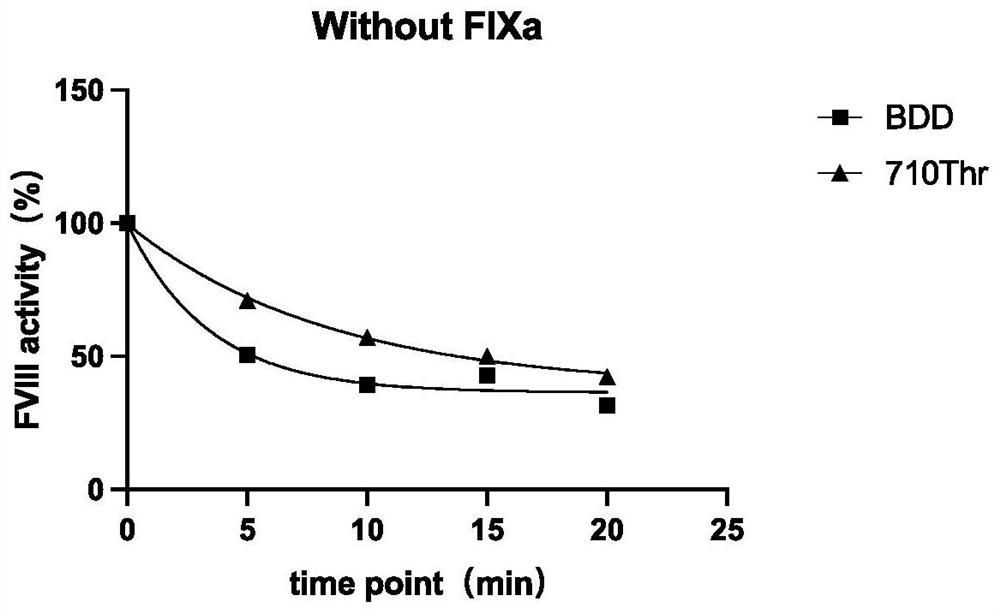
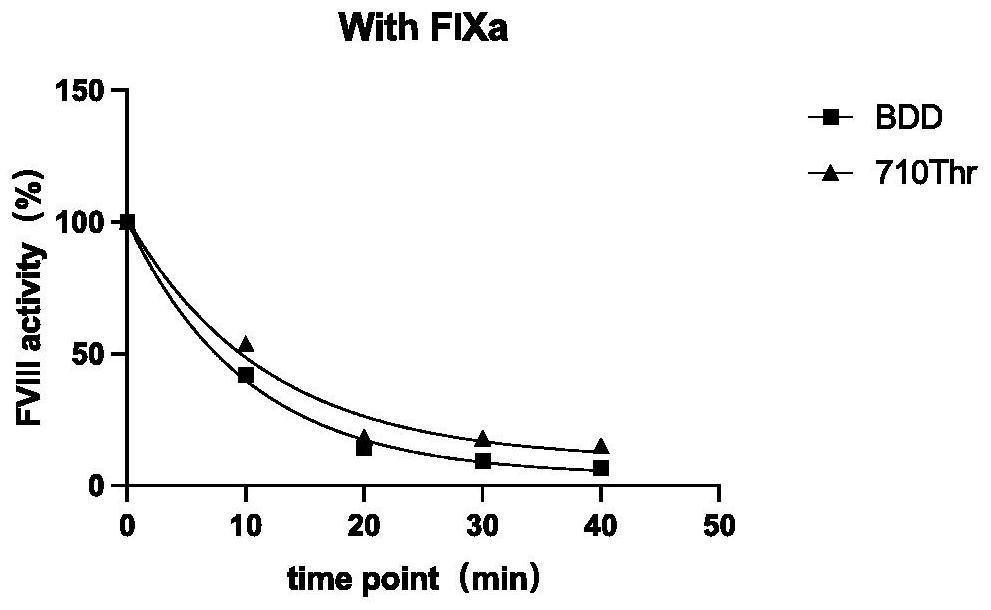
[0027] A mutant protein of highly active coagulation factor VIII or VIIIa polypeptide variant Gly710Thr, the amino acid sequence of which is shown in any of SEQ ID NOs: 21-29, the amino acid of the mutant at position 710 is Thr rather than wild-type VIII or VIIIa Gly . This example takes the mutant protein of hFVIII cDNA as an example.
[0028] 1. Expression and purification of FVIII Gly710Thr mutant
[0029] (1) Expression: use QuikChange site-directed mutagenesis kit ( IIXL Site-DirectedMutagenesis Kit, Agilent, USA), using human B region deletion coagulation factor VIII (BDD-hFVIII) as a template, using PCR site-directed mutagenesis to introduce a mutation site in wild-type FVIII, and replace 710Gly with 710Thr , the amplified product was transformed by Dpn I digestion (5-10 μl of the mutant product after Dpn I digestion was added to each 100 μl of competent bacteria), and the transformed bacteria were coated to contain 1 / 2000 ampicillin The clones were obtained by cult...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com