Highly-effectively copper-based catalyst for decomposing methanol to produce hydrogen
A copper-based catalyst, methanol decomposition technology, applied in catalyst activation/preparation, metal/metal oxide/metal hydroxide catalyst, heterogeneous catalyst chemical elements, etc., can solve the problem of narrow catalyst reaction temperature zone and catalyst stability Deterioration, poor catalyst selectivity and other problems, to achieve the effect of good low temperature activity, easy washing and long service life
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
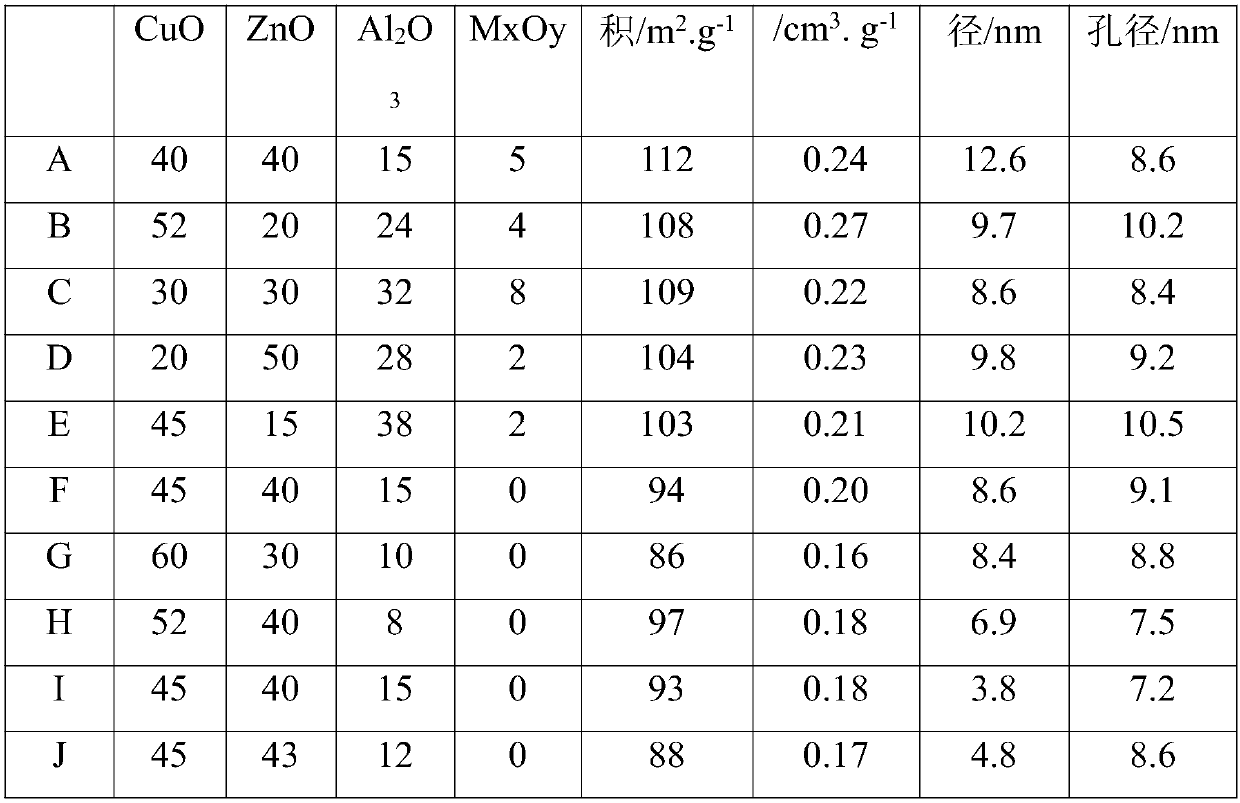
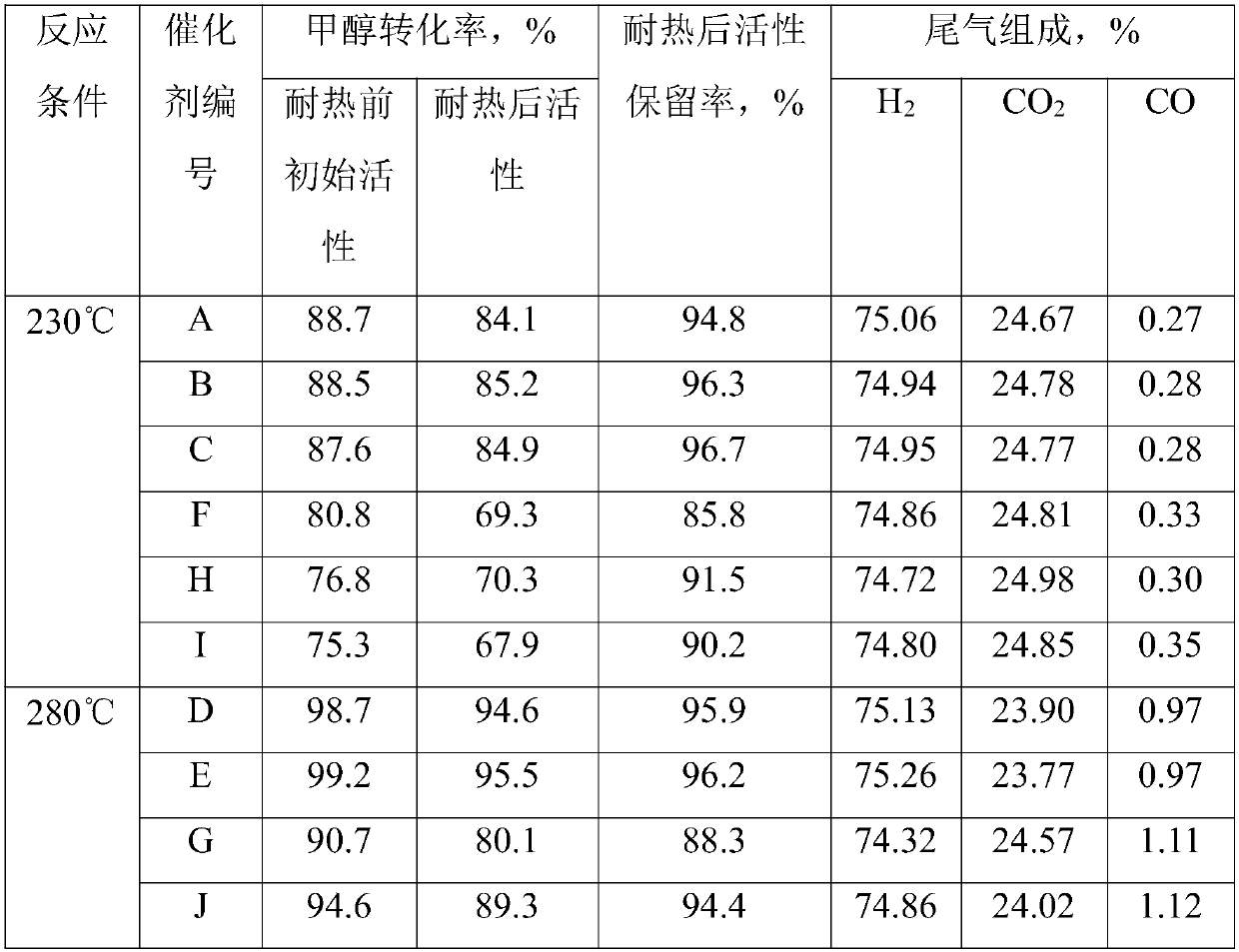
Embodiment 1
[0052] 110g aluminum nitrate (Al(NO 3 ) 3 9H 2 O) and 32g magnesium nitrate (Mg(NO 3 ) 2 ·6H 2 O) adding 415mL deionized water to make molar concentration is 1mol / L mixed salt solution A; 123g copper nitrate (Cu(NO 3 ) 2 ·3H 2 O), 148g zinc nitrate (Zn(NO 3 ) 2 ·6H 2 O) add 1.0L deionized water to form solution B; Take by weighing 190g sodium carbonate (Na 2 CO 3 ) was dissolved in 1.5L of water to form solution C.
[0053] Put solution A and 0.4L of solution C into the precipitation container at the same time under stirring, add 100mL deionized water into the container in advance, and heat it to 35°C, control the temperature of the reaction process at 35°C, and control the reaction pH value at about 8.5 , aged under reaction conditions for 3h after precipitation. After aging, add 0.5 mol of methanol to it and raise the temperature to 40°C. Solution B and the remaining 1.1L solution C are simultaneously dripped into the precipitation container under stirring. The ...
Embodiment 2
[0056] 170g aluminum nitrate (Al(NO 3 ) 3 9H 2 O) and 26g magnesium nitrate (Mg(NO 3 ) 2 ·6H 2 O) adding 550mL deionized water to make molar concentration is 1mol / L mixed salt solution A; 157g copper nitrate (Cu(NO 3 ) 2 ·3H 2 O), 75g zinc nitrate (Zn(NO 3 ) 2 ·6H 2 O) add 0.8L deionized water to form solution B; Take by weighing 270g potassium carbonate (K 2 CO 3 ) was dissolved in 1.5L of water to form solution C.
[0057] Put solution A and 0.7L of solution C into the precipitation container while stirring, add 100mL of deionized water in advance, and heat it to 50°C. The temperature of the reaction process is controlled at 50°C, and the reaction pH value is controlled at 7.5 After precipitation, it was aged for 3h under the reaction conditions. After aging, add 0.98 mol of ethanol to it, and raise the temperature to 60°C. Solution B and 0.7L of solution C are simultaneously dripped into the precipitation container under stirring. The temperature of the reactio...
Embodiment 3
[0060] 235g aluminum nitrate (Al(NO 3 ) 3 9H 2 O) and 23g calcium nitrate (Ca(NO 3 ) 2 ) was added with 770mL deionized water to form a molar concentration of 1mol / L mixed salt solution A; 93g copper nitrate (Cu(NO 3 ) 2 ·3H 2 O), 112g zinc nitrate (Zn(NO 3 ) 2 ·6H 2 O) add 1.0L deionized water to form solution B; Take by weighing 106g sodium carbonate (Na 2 CO 3 ) and 164g sodium bicarbonate (NaHCO 3 ) was dissolved in 1.5L of water to form solution C.
[0061] Put solution A and 1L of solution C into the sedimentation container while stirring, add 100mL deionized water in advance, and heat it to 600°C. The temperature of the reaction process is controlled at 60°C, and the reaction pH value is controlled at about 6.5 , aged under reaction conditions for 3h after precipitation. After aging, add 0.4mol propanol to it, and raise the temperature to 80°C, and drop solution B and 0.5L solution C into the precipitation container simultaneously under stirring, control th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com