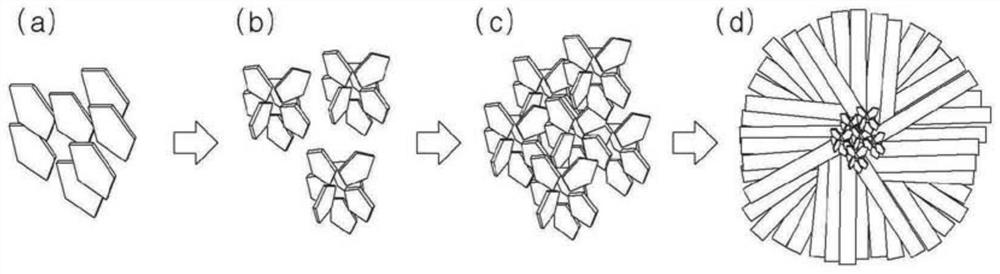
Method for preparing positive electrode active material precursor for lithium secondary battery, positive electrode active material precursor, and positive electrode active material, positive electrode, and lithium secondary battery prepared by using same
A technology for cathode active materials and precursors, applied in the field of preparing cathode active material precursors for lithium secondary batteries, cathode active material precursors, and cathode active materials prepared by using the precursors, cathodes, and lithium secondary batteries , can solve problems such as poor thermal stability, battery rupture, fire, etc., and achieve the effect of improving electrochemical performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0137] NiSO was added in an amount such that the molar ratio of nickel:cobalt:manganese was 88:5:7 4 , CoSO 4 and MnSO 4 Mix in water to prepare an aqueous transition metal solution (first step).
[0138] The vessel containing the aqueous transition metal solution, additional NaOH solution, and NH 4 The aqueous OH solutions were each connected to a 350 L continuous filter tank reactor (CFTR) equipped with a filtration device (filter).
[0139] Subsequently, after 86 L of deionized water was put into the reactor, dissolved oxygen in the water was removed by purging the reactor with nitrogen at a rate of 20 L / min to create a non-oxidizing atmosphere in the reactor. After that, after adding NaOH aqueous solution and NH 4 After the OH aqueous solution, while stirring the mixture at a stirring speed of 700 rpm, a reaction mother liquor having a pH of 11.7 to 11.9 was prepared (second step).
[0140] After that, the transition metal aqueous solution, NH 4 Aqueous OH and NaOH wer...
Embodiment 2
[0146] In the same manner as in Example 1, except that the precursor core formation step (third step) and the particle growth reaction step (fourth step) were carried out for a total of 53 hours and the stabilization reaction (fifth step) was carried out for 15 hours A cathode active material precursor was prepared.
experiment example 1
[0157] Experimental Example 1: Particle Characterization
[0158] (1) Grain size of precursor
[0159] The grain sizes of the positive electrode active material precursor particles prepared in Examples 1 and 2 and Comparative Examples 1 to 2 were measured by the following method.
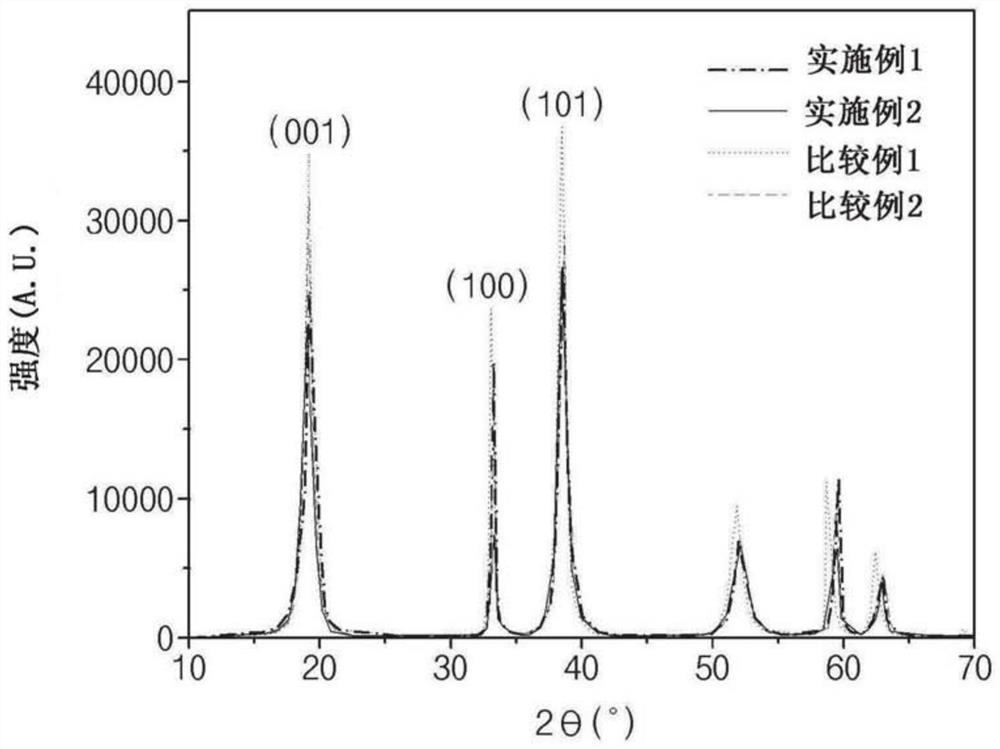
[0160] The X-ray diffraction (XRD) patterns of the precursors prepared in Examples 1 and 2 and Comparative Examples 1 and 2 were measured using an X-ray diffraction analyzer (Rigaku Corporation). The XRD patterns of the positive electrode active material precursor particles prepared in Examples 1 and 2 and Comparative Examples 1 and 2 are shown in figure 2 middle.
[0161] After obtaining the half-value widths of the peaks of the respective crystal planes from the measured XRD patterns, the grain sizes in the respective crystal planes of the precursor were calculated by ellipsoid modeling using the Scherrer formula.
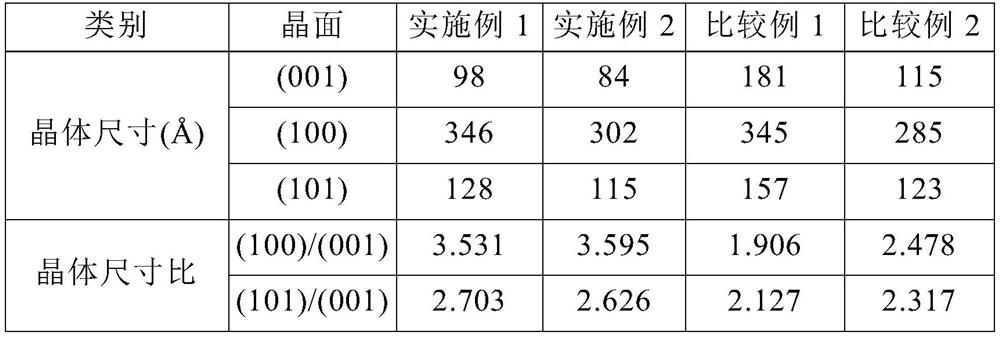
[0162] The measurement results are shown in Table 1 below.
[0163] [Table 1]...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com