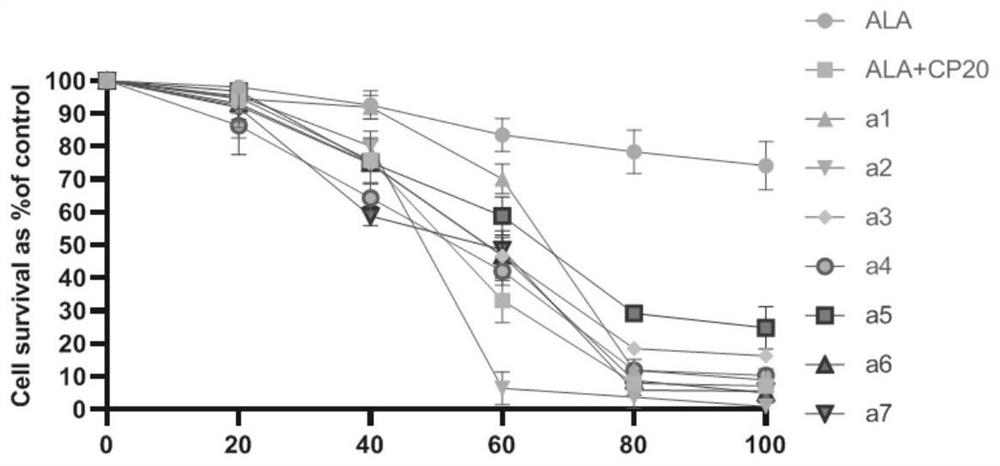
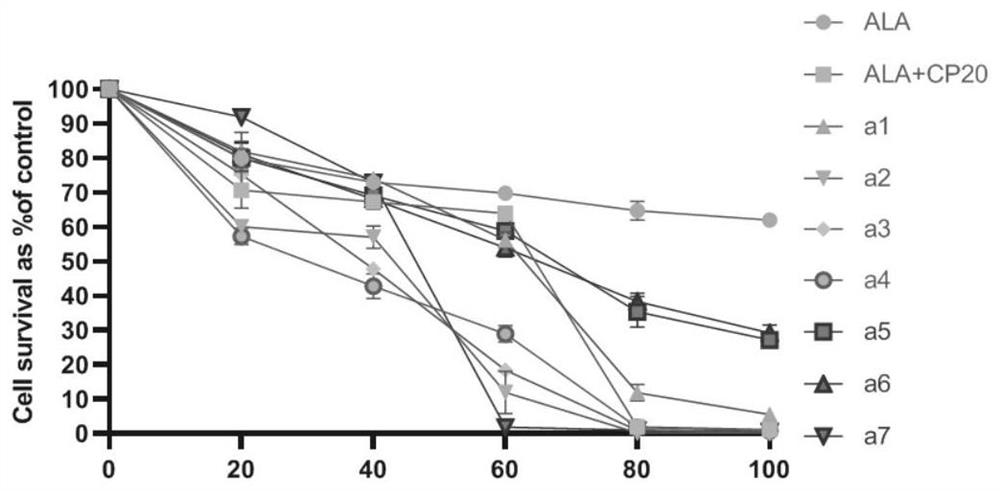
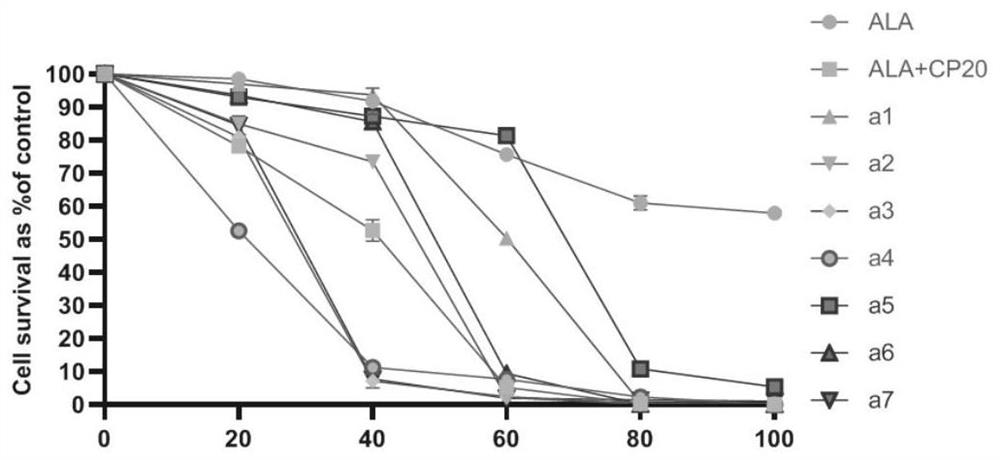
ALA hybrid 3-hydroxypyridone derivative as well as preparation method and application thereof
A technology for hydroxypyridone and derivatives, which is applied in the field of ALA hybrid 3-hydroxypyridone derivatives and its preparation, and can solve the problems of decreased therapeutic effect and poor pharmacokinetic performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0114] (1) ALA hydrochloride (4.20 g, 25.0 mmol) and NaHCO were added to a 250 mL round-bottomed flask 3 (12.60g, 150mmol) and Boc 2 O (6.00 g, 27.5 mmol), and 75 mL of anhydrous methanol was added as a solvent, stirred at room temperature under argon protection, and the reaction progress was detected by TLC during the reaction, and the color was developed in an iodine cylinder. After 24h, the reaction was stopped, and the reaction solution was beige at this time, and the unreacted NaHCO was 3 The solid was removed by filtration with a Buchner funnel and rinsed with methanol. The filtrate was distilled under reduced pressure and dissolved in 30 mL of water, then acidified to pH=1~2 with 10% potassium hydrogen sulfate solution, and extracted with ethyl acetate for 3 times. , the organic layers were combined and washed with saturated brine (3×25 mL), dried over anhydrous sodium sulfate for 12 h, filtered to remove the solid, evaporated to remove the solvent under reduced pressu...
Embodiment 2
[0126] (1) In a 100 mL round-bottomed flask, add the yellow oil (3.30 g, 10.0 mmol) and amylamine (5.23 g, 60.0 mmol) obtained in Example 1 (6), ethanol and water (V:V=1: 1) 20 mL was used as a solvent, reacted under reflux conditions for 18 h, during which TLC was used to monitor the reaction progress, after the reaction was completed, the solvent was distilled off under reduced pressure to obtain a brown oily substance, dissolved in ethanol and adjusted to pH=1 with concentrated hydrochloric acid, continued to reflux for 4 h, TLC The reaction progress was monitored, after the reaction was completed, it was cooled to room temperature, the solvent was distilled off under reduced pressure, the residue was dissolved in water and washed twice with diethyl ether, then adjusted to pH=9 with 10 mol / L sodium hydroxide solution, extracted with dichloromethane (3 × 20 mL), combined the organic layers, dried over anhydrous sodium sulfate, filtered, evaporated under reduced pressure to re...
Embodiment 3
[0133] (1) In a 100 mL round-bottomed flask, add the yellow oil (3.30 g, 10.0 mmol) and n-hexylamine (6.07 g, 60.0 mmol) obtained in Example 1 (6), ethanol and water (V:V=1: 1) 20 mL was used as a solvent, reacted under reflux conditions for 18 h, during which TLC was used to monitor the reaction progress, after the reaction was completed, the solvent was distilled off under reduced pressure to obtain a brown oily substance, dissolved in ethanol and adjusted to pH=1 with concentrated hydrochloric acid, continued to reflux for 4 h, TLC The reaction progress was monitored, after the reaction was completed, it was cooled to room temperature, the solvent was distilled off under reduced pressure, the residue was dissolved in water and washed twice with diethyl ether, then adjusted to pH=9 with 10 mol / L sodium hydroxide solution, extracted with dichloromethane (3 × 20 mL), combined the organic layers, dried over anhydrous sodium sulfate, filtered, evaporated under reduced pressure to...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com