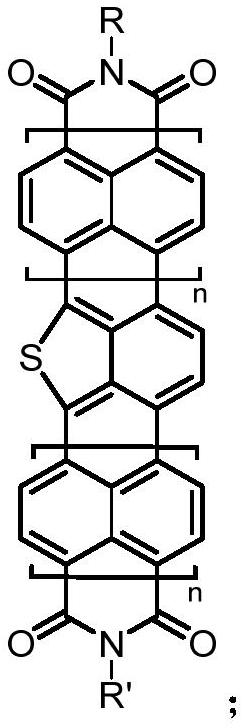
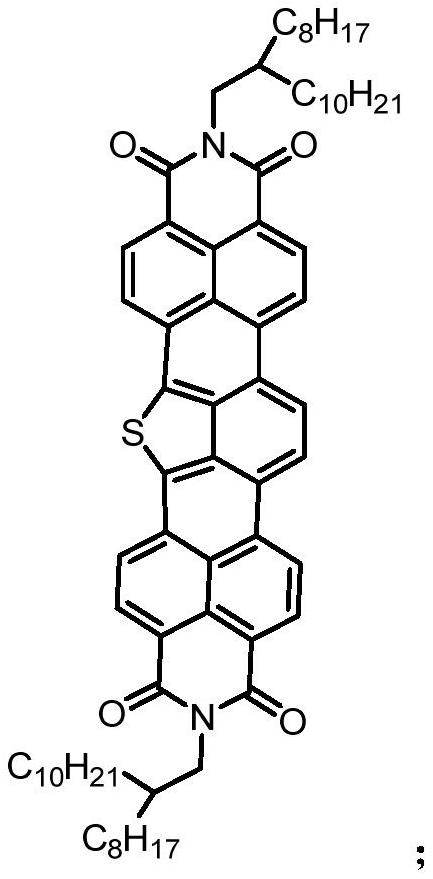
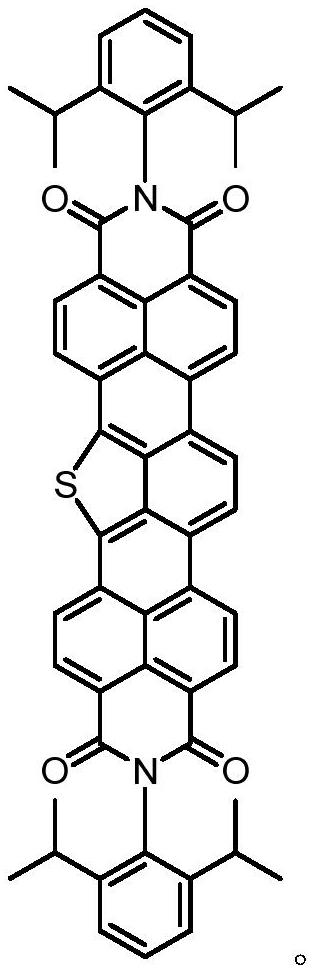
Diimide derivative containing isothioindene and preparation method thereof
A technology of imide and derivative is applied in the field of isothianaline-containing imide derivative and preparation thereof, can solve problems such as difficulty in synthesis, achieve low cost of raw materials, lower LUMO energy level, and large conjugation system effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0053] A kind of imide derivative containing isothiane, the concrete preparation method is as follows:
[0054] (1) Put 1 g of compound 1 (6.57 mmol) into a dry three-necked flask, evacuate for 10-15 minutes, add 100 mL of dry tetrahydrofuran with a syringe, transfer it into a -78°C ethanol ice bath, and stir for 15 min to obtain a uniform solution, 2.75 g of bis(trimethylsilyl) lithium amide (16.42 mmol) was added dropwise to the solution with a syringe, the temperature was slowly raised to 25 °C, stirred for 3 h, and deoxygenated to obtain compound 2. Compound 2 did not need to be purified. used for the next reaction.
[0055] The reaction formula is as follows:
[0056]
[0057] (2) 600mg of compound 2 (4.47mmol) was dissolved in 100mL of tetrahydrofuran, transferred to -78°C ethanol ice bath, and 1.30g of N,N,N',N'-tetramethylethylenediamine (11.18mmol) was added with a syringe , stirred for 15 min, then added 4.11 mL of n-butyllithium (10.28 mmol) dropwise with a syr...
Embodiment 2
[0076] A kind of imide derivative containing isothiane, the concrete preparation method is as follows:
[0077] (1) 500mg of compound 1 (1.09mmol), 1.19g of compound 2 (2.72mmol) and 62.83mg of tetrakis(triphenylphosphine)palladium (0.109mmol) were added to the reaction tube successively, protected from light, and evacuated for 10-15min , and then 20 mL of toluene was added to the reaction tube with a syringe, the temperature was raised to 110 ° C and the reaction was performed overnight, the product was extracted three times with dichloromethane, the organic phases were combined, dried with anhydrous sodium sulfate, and the solvent was removed by rotary evaporation. A mixture of petroleum ether (dichloromethane and petroleum ether in a volume ratio of 1:2) was used as the eluent to carry out silica gel column chromatography separation to obtain product 3 in a yield of 25%.
[0078] The reaction formula is as follows:
[0079]
[0080] (2) Dissolve 2.66g of potassium hydro...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com