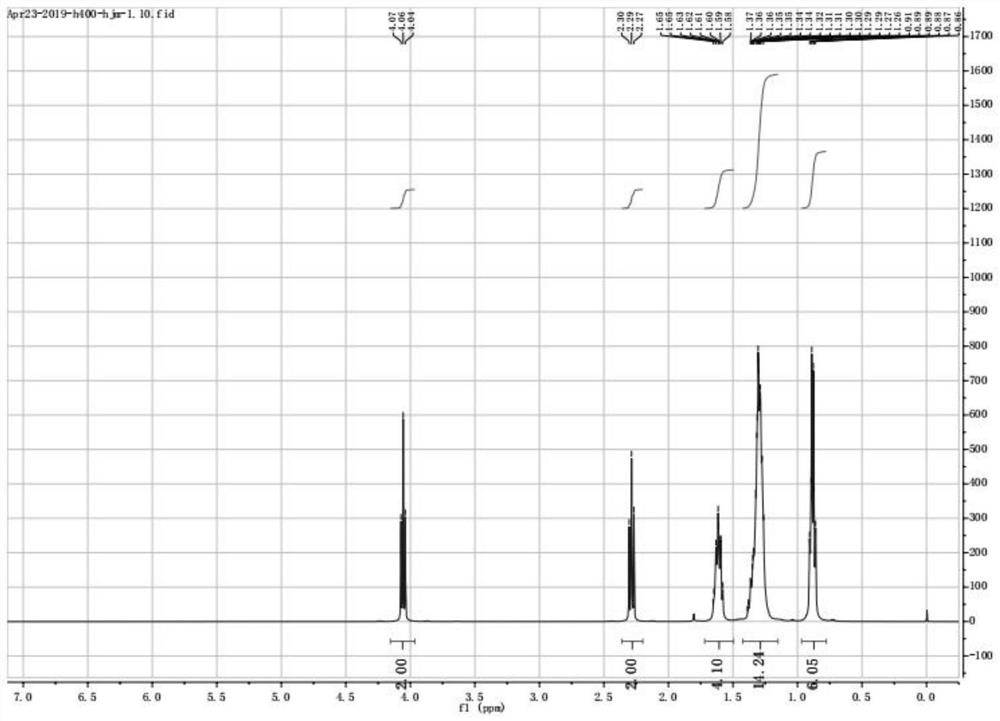
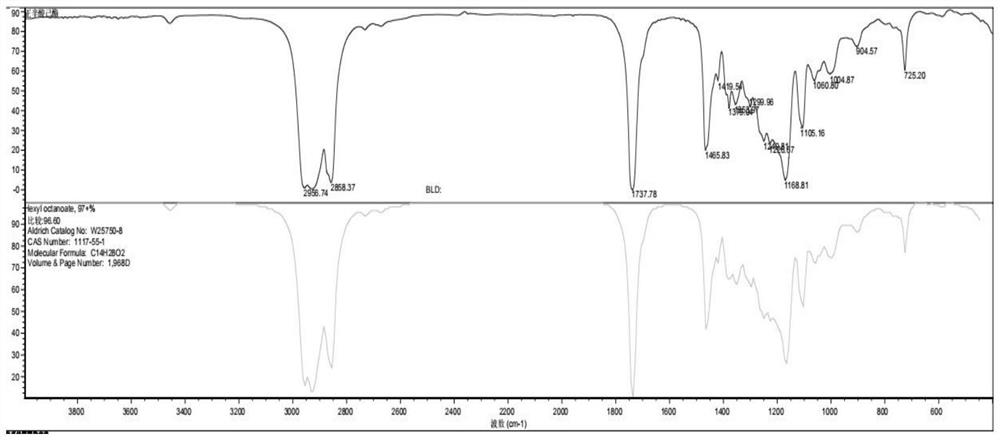
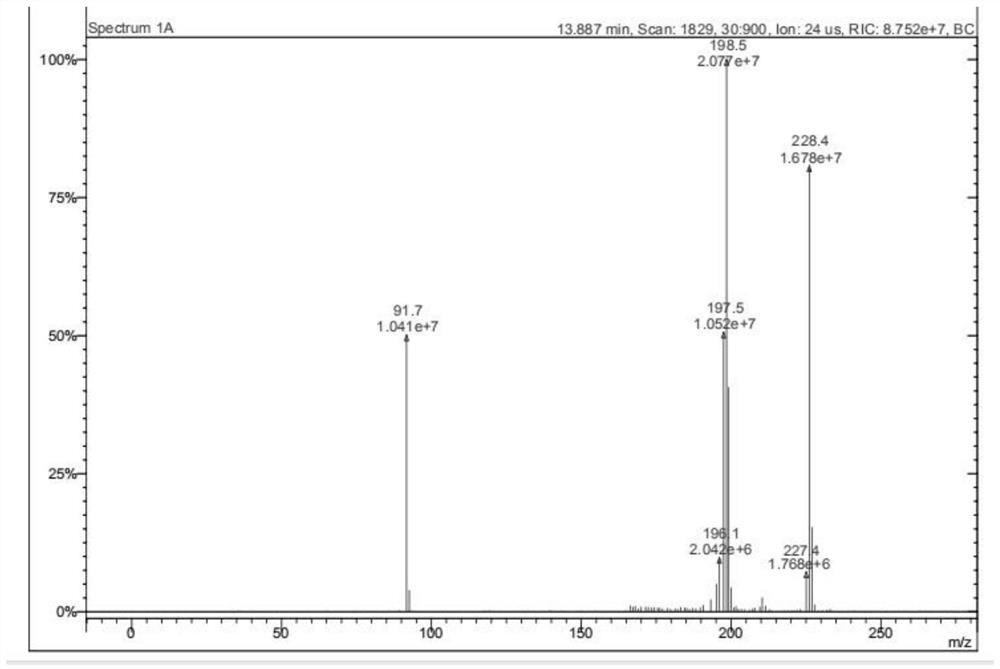
Preparation method of hexyl n-caprylate
A technology of n-octanoic acid and hexyl ester, applied in the field of preparation of n-octanoic acid hexyl ester, can solve the problems of low yield of n-octanoic acid hexyl ester and high corrosion of equipment, and achieve the advantages of improving selectivity, increasing yield and reducing corrosiveness. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0023] The invention provides a preparation method of hexyl n-octanoate, comprising:
[0024] After mixing n-octanoic acid, n-hexanol and tetrabutyl titanate, esterification reaction was performed to obtain hexyl n-octanoate.
[0025] In the present invention, n-octanoic acid and n-hexanol are preferably mixed and heated, and then tetrabutyl titanate is added to carry out an esterification reaction to obtain hexyl n-octanoate.
[0026] In the present invention, the ratio of the substance amounts of the n-octanoic acid and n-hexanol is preferably 1:(1.5-5), more preferably 1:(2-3). The present invention limits the amount of n-hexanol to be more than that of n-octanoic acid, so on the one hand, the reaction of n-octanoic acid is guaranteed to be complete; .
[0027] In the present invention, the temperature reached by the heating is preferably 110 to 130°C, and more preferably 120°C. In the present invention, the temperature to which the heating is performed is the temperatur...
Embodiment 1
[0038] 1. Synthesis reaction
[0039] 1.1 Feeding: add 291.4g of n-octanoic acid and 417g of n-hexanol in a 4-port glass reactor of 5L; feed nitrogen into the reaction mass for 1 minute, and after being heated to 120° C., add a catalyst (mass concentration is 10% tetramethyl titanate). n-hexanol solution of butyl ester) 4g, continue to heat up to 180 ℃, keep warm until no water droplets are produced in the oil-water separator, sample and analyze the acidity value, stop heating when the acidity is less than 0.1%, and terminate the reaction. (The material ratio of n-octanoic acid and n-hexanol is 1:2, and the mass of tetrabutyl titanate accounts for 0.056% of the total weight of the starting materials n-octanoic acid and n-hexanol)
[0040] 2. Purification
[0041] 2.1 Negative pressure dealcoholization
[0042] After the reaction, the crude product is subjected to negative pressure distillation to remove unreacted n-hexanol, the vacuum degree is controlled between 0.2 and 0.5...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com