Nano preparation of porous material coated alkynyl modified sorafenib derivative and silver, preparation method and application thereof
A technology of sorafenib and nano-preparation, applied in the directions of nanotechnology, nanotechnology, nanomedicine, etc., can solve problems such as increasing the survival time of patients, and achieve a good synergistic anti-tumor effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
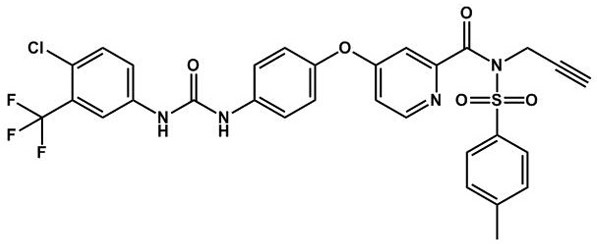
[0032] Synthesis of Alkynyl Modified Sorafenib Derivatives:
[0033] (1) Preparation of carboxylic acid intermediates
[0034] Sorafenib (3 g) and NaOH (3.87 g) were dissolved in 20 mL absolute ethanol solution, N 2 protection, and the reaction was refluxed at 80 °C for 8 h. After the reaction was completed, the organic solvent was removed by low pressure rotation, dilute hydrochloric acid (2 mol / L) was added to neutralize the excess alkali, the pH was adjusted to about 5, the color of the reaction solution changed from blue to light pink, and a gray solid was precipitated after continuing the reaction for 5h, Suction filtration with a Buchner funnel, washed with a small amount of distilled water, and dried in a vacuum drying oven at 40 o Dry at C overnight to obtain 4-(4-(3-(4-chloro-3-(trifluoromethyl)phenyl)ureido)phenoxy)picolinic acid (referred to as carboxylic acid intermediate) as a grey solid powder. .
[0035] (2) Preparation of acid chloride intermediates
[003...
Embodiment 2
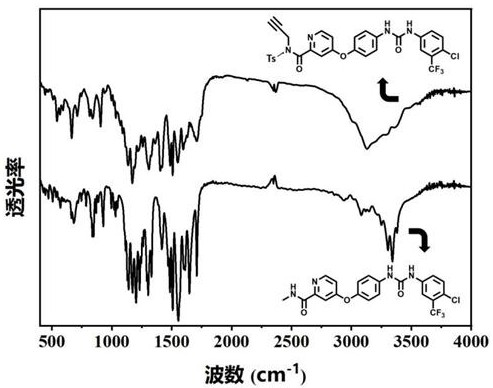
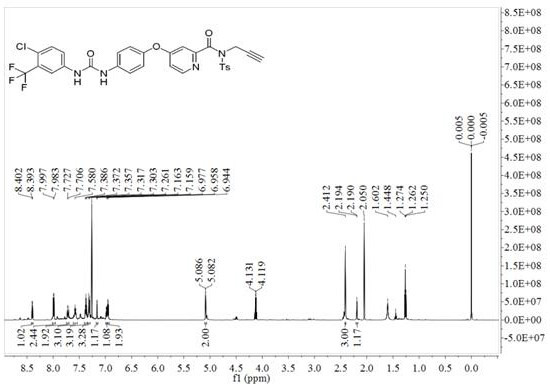
[0042] Example 2 Preparation method of ZIF-8-encapsulated alkynyl-modified sorafenib derivatives (ASOR@ZIF-8 for short)
[0043] The preparation method of described ASOR@ZIF-8 is as follows:
[0044] With zinc nitrate and 2-methylimidazole, deionized water molar ratio 1: 25: 700, stir at room temperature to obtain white emulsion; In the obtained emulsion, add the alkynyl group prepared in Example 1 The modified sorafenib derivative was continuously stirred until uniform to obtain a suspension; the obtained suspension was transferred to a hydrothermal synthesis reactor, and the hydrothermal reaction was carried out at 130 °C for 1.5 h. After cooling, centrifugation, washing with ethanol solution, and drying, a white solid was obtained, which was ASOR@ZIF-8. Elemental analysis showed that the mass percentage of ASOR was 12.6%.
[0045] The molar ratio of the alkynyl-modified sorafenib derivative to the sum of the molar numbers of zinc nitrate, 2-methylimidazole and deionized wa...
Embodiment 3
[0047] Example 3 ZIF-8 encapsulated alkynyl-modified sorafenib derivatives and silver nanoformulations (ASOR-Ag for short) x @ZIF-8) preparation method
[0048] The ASOR-Ag x The preparation method of @ZIF-8 is as follows: Disperse 50 mg of the ASOR@ZIF-8 nanomaterial prepared in the above Example 2 in 10 mL of methanol solution with a concentration of 0.025 mol / L silver nitrate, stir and react for 6 h (stirring speed 400 rpm), centrifuged (10,000 rpm), washed with ethanol solution, and dried to obtain a white powder, which is ASOR-Ag x @ZIF-8, elemental analysis showed an ASOR mass percentage of 7.5%.
[0049] ASOR-Ag x TEM image of @ZIF-8 ( Figure 5 ) analysis showed that ASOR-Ag x Although @ZIF-8 maintains the approximate configuration of ZIF-8, the edges and corners of the polyhedral structure are completely blurred, and the size is comparable to that of ASOR@ZIF-8 (80~120 nm), and Ag can be clearly observed inside the ZIF-8 framework. presence of nanoparticles.
PUM
| Property | Measurement | Unit |
|---|---|---|
| size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com