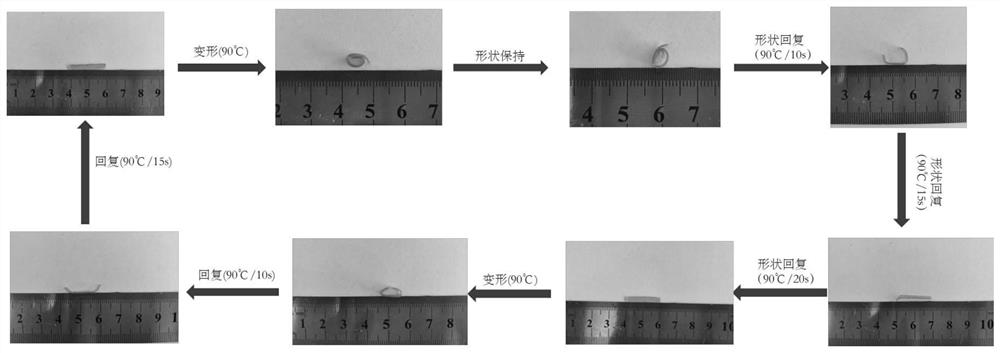
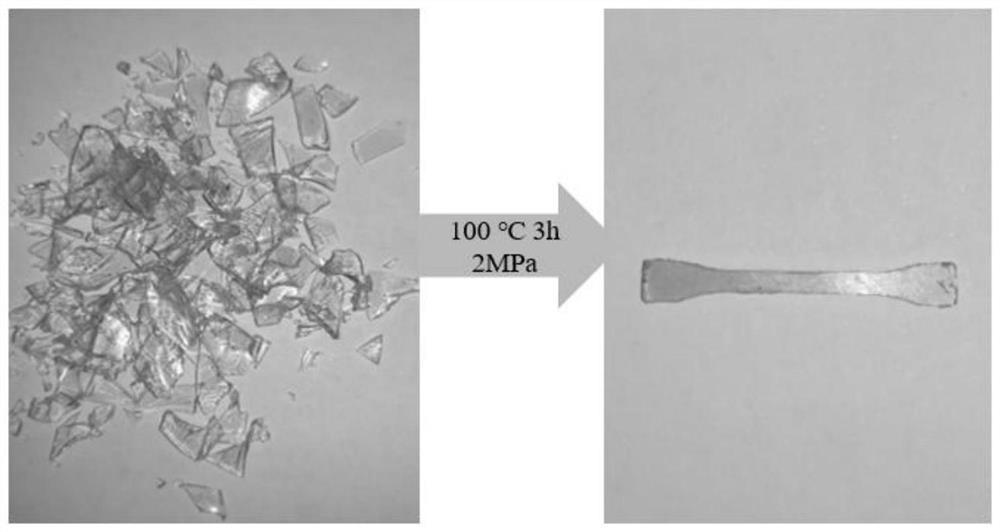
Non-isocyanate-based polyurethane monomer, thermosetting non-isocyanate-based shape memory polyurethane and preparation and recycling methods of non-isocyanate-based polyurethane monomer and thermosetting non-isocyanate-based shape memory polyurethane
A non-isocyanate and polyurethane monomer technology, applied in the direction of organic chemistry, can solve the problems of no recycling performance and poor mechanical properties, and achieve good shape recovery rate, improved mechanical strength, and good shape memory performance.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0033] The present invention provides the preparation method of the non-isocyanate-based polyurethane monomer described in the above technical solution, comprising the following steps:
[0034] The non-isocyanate-based polyurethane monomer is obtained by mixing the cyclic carbonate with double bond, thiol and photoinitiator under ultraviolet light irradiation to carry out a click reaction.
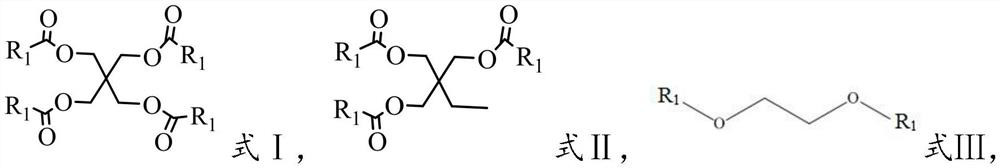
[0035] In the present invention, the cyclic carbonate with double bond is ethylene ethylene carbonate (4-Vinyl-1,3-dioxolan-2-one) or has the structure shown in formula IV, in formula IV, R 3 is -H or -CH 3 :
[0036]
[0037] The present invention has no special requirements on the source of the cyclic carbonate with double bonds, and can be prepared by using commercially available products well-known to those skilled in the art or by using a preparation method well-known to those skilled in the art; in the present invention , when the cyclic carbonate with double bond has the struct...
Embodiment 1
[0056] A kind of non-isocyanate group polyurethane monomer, preparation method is as follows:
[0057] Mix 2.7g of ethylene ethylene carbonate, 2.9g of pentaerythritol tetra-3-mercaptopropionate and 60mg of photoinitiator (2,2-dimethoxy-2-phenylacetophenone) and mix well. The liquid is placed under an ultraviolet lamp (the wavelength of ultraviolet light is 365nm, and the irradiation intensity is 30mw / cm -2 ) reaction 10min, obtain the monomer with four five-membered cyclic carbonates, i.e. non-isocyanate based polyurethane monomer, the reaction formula is as follows:
[0058]
[0059] A thermosetting non-isocyanate-based shape memory polyurethane, the preparation method is as follows:
[0060] Add 15 mL of DMF solvent to the above-mentioned non-isocyanate-based polyurethane monomer (with four five-membered cyclic carbonates) and mechanically stir until uniformly mixed to obtain a non-isocyanate-based polyurethane monomer solution; 2.043 g of isophorone di The amine was a...
Embodiment 2
[0063] A kind of non-isocyanate group polyurethane monomer, preparation method is as follows:
[0064] 2.8 g of hydroxymethyldioxolane, 1.9 g of dimethacrylic anhydride and 47 mg of toluenesulfonic acid were mixed in N 2 The cyclic carbonate with double bonds was synthesized by reacting at 135 °C for 6 h; the obtained cyclic carbonate with double bonds, 2.1 g of 3,6-dioxa-1,8-octanedithiol and 60 mg of The photoinitiator (2,2-dimethoxy-2-phenylacetophenone) is mixed evenly, and the mixed liquid is placed under an ultraviolet lamp (the wavelength of ultraviolet light is 365nm, and the irradiation intensity is 30mw / cm -2 ) reaction 5min, synthesizing the monomer with bicyclic carbonate, i.e. non-isocyanate based polyurethane monomer, the reaction formula is as follows:
[0065]
[0066] A thermosetting non-isocyanate-based shape memory polyurethane, the preparation method is as follows:
[0067] Add 15 mL of DMF solvent to the above-mentioned non-isocyanate-based polyuretha...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
| glass transition temperature | aaaaa | aaaaa |
| glass transition temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com