Butylphthalide-oxadiazole/thiadiazole compound as well as preparation method and application thereof
A technology of thiadiazoles and compounds, applied in the field of pharmacy, can solve the problems of low overall curative effect and limited use, and achieve excellent anti-platelet aggregation activity in vitro
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] of 3-butyl-6-((5-(isopropylsulfoxide)-1,3,4-oxadiazol-2-yl)methoxy)isobenzofuran-1(3H)-one The preparation method includes the following steps:
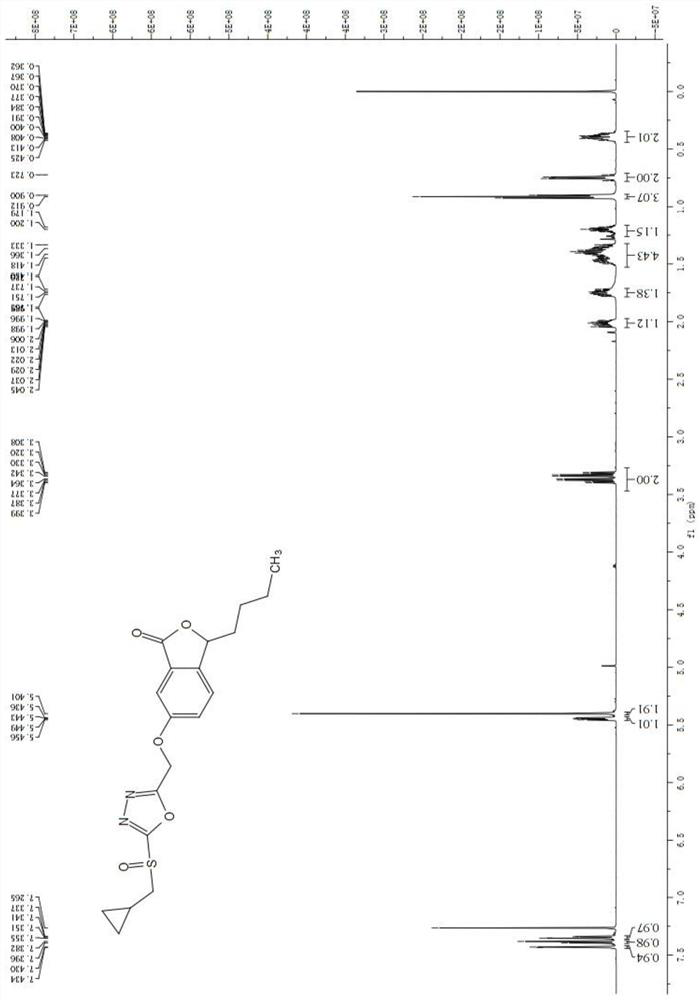
[0034] (1) Synthesis of 3-butyl-6-nitroisobenzofuran-1(3H)-one (Compound 1): butylphthalide (10 g, 52.6 mmol) was dissolved in 40 mL of 98% H 2 SO 4 Medium, at 0-5℃, add KNO in batches 3 (6.9 g, 67.6 mmol), stirred at room temperature for 3 h; TLC detected the completion of the reaction, added ice-water mixture (150 mL), extracted three times with ethyl acetate (3×300 mL), washed with saturated brine (100 mL), Anhydrous Na 2 SO 4 After drying, concentration, and silica gel column chromatography with PE:EA=10:1, compound 1 (9.3 g, 75.2%) was obtained as a pale yellow liquid; 1 HNMR (400MHz, CDCl 3 )δ8.72(d,1H,J=2.0Hz),8.57(dd,1H,J=8.4,2.0Hz),7.68(d,1H,J=8.4Hz),5.62-5.59(m,1H), 2.16-2.08(m, 1H), 1.87-1.78(m, 1H), 1.45-1.36(m, 4H), 0.94(t, 3H, J=6.8Hz, CH 3 ); 13 C NMR (100MHz, CDCl 3 )δ168.0,155.3,149.0,128.8,127.9,123...
Embodiment 2
[0043]3-Butyl-6-((5-(cyclopropylmethyl)sulfoxide)-1,3,4-oxadiazole-2-methoxy)isobenzofuran-1(3H)-one Preparation of:
[0044] Identical to the preparation procedure of embodiment 1, the difference is only that the isopropyl bromide in the step (7) is replaced with the cyclopropyl bromide of the amount of equivalent substance;
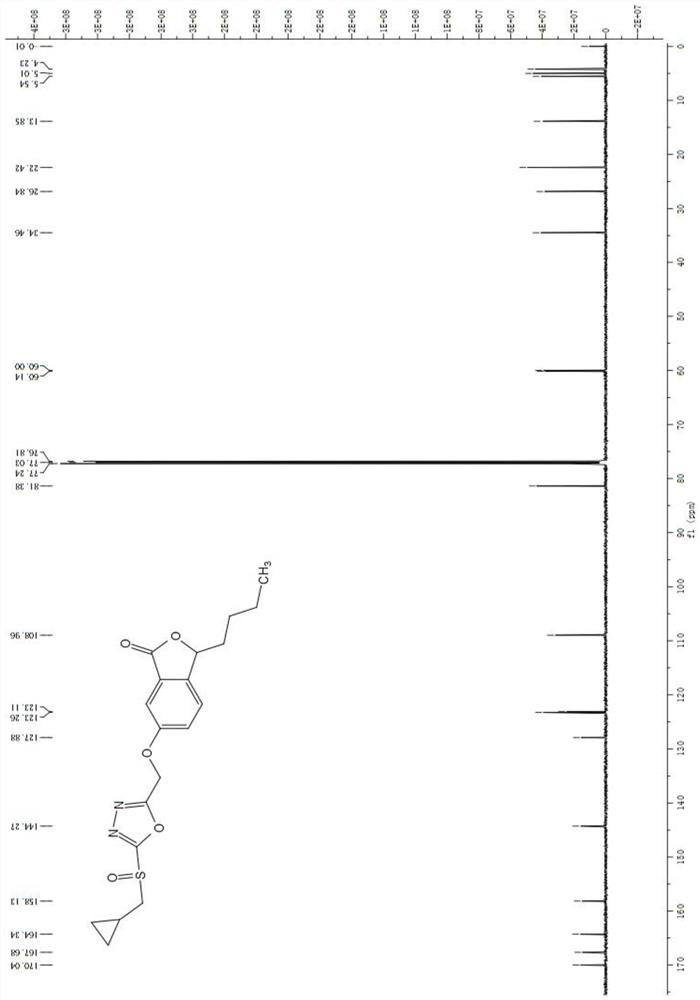
[0045] 3-Butyl-6-((5-(cyclopropylmethylthio)-1,3,4-oxadiazol-2-yl)methoxy)isobenzofuran-1(3H)-one (7b): white solid, yield 74.2%; m.p.74-75°C; 1 H NMR (600MHz, CDCl 3 )δ7.43(d,J=2.0Hz,1H),7.37(d,J=8.4Hz,1H),7.34(dd,J=8.4,2.4Hz,1H),5.44(dd,J=7.8,4.0 Hz, 1H), 5.27(s, 2H), 3.23(d, J=7.4Hz, 2H), 2.03-1.98(m, 1H), 1.80-1.66(m, 1H), 1.52-1.32(m, 4H) ,1.31-1.16(m,1H),0.91(t,J=7.0Hz,3H),0.70-0.64(m,2H),0.40-0.34(m,2H); 13 CNMR (150MHz, CDCl 3 )δ170.2,166.7,162.4,158.4,143.9,127.8,123.0,109.1,81.3,60.1,38.7,34.5,26.8,22.4,13.9,10.5,6.2; HRMS(ESI)calcd for C 19 H 23 N 2 O 4 S[M+H] + m / z:375.1379,found 375.1374;
[0046] 3-Butyl-6-((5-(cyclopropylmethy...
Embodiment 3
[0048] 3-butyl-6-(5-benzylsulfoxide)-1,3,4-oxadiazole-2-methoxy)-3-butylbenzofuranone-1(3H)-one preparation:
[0049] The same as the preparation step of Example 1, the difference is only that the isopropyl bromide in the step (7) is replaced by the benzyl bromide of an equivalent amount;
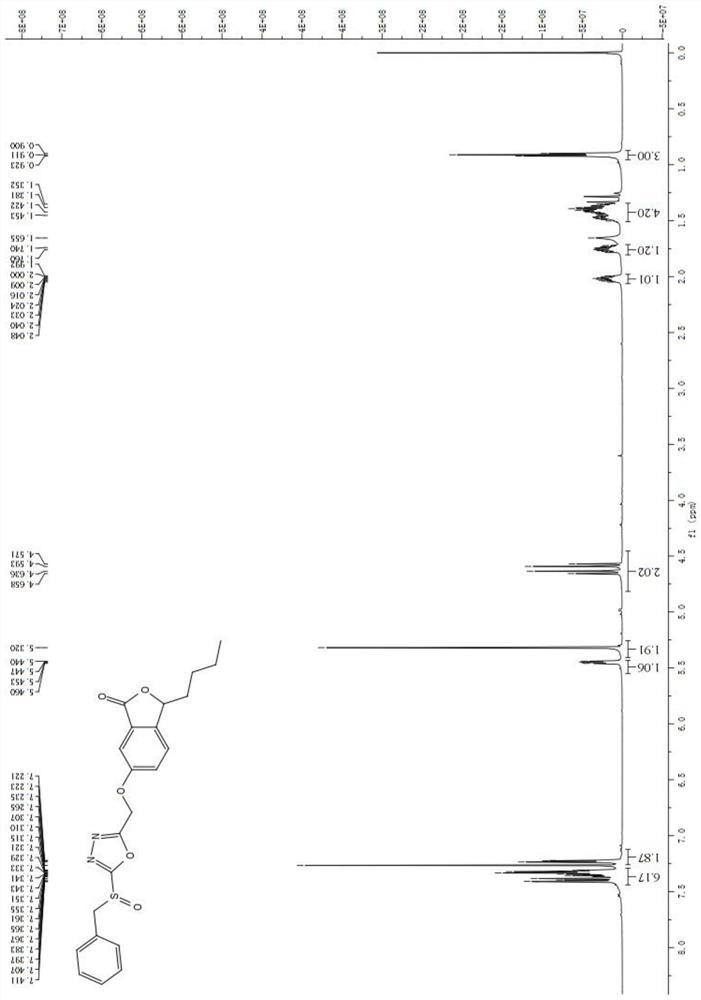
[0050] 6-(5-(Benzylthio)-1,3,4-oxadiazol-2-yl)methoxy-3-butylbenzofuran-1(3H)-one (7c): colorless liquid , the yield is 73.1%; 1 H NMR (400MHz, CDCl 3 )δ7.46-7.42(m,3H),7.41-7.31(m,5H),5.45(dd,J=7.8,4.2Hz,1H),5.28(s,2H),4.51(s,2H),2.07 -1.99(m,1H),1.82-1.68(m,1H),1.47-1.38(m,4H),0.95-0.91(m,3H); 13 C NMR (100MHz, CDCl 3 )δ170.2,162.6,158.4,144.0,135.2,129.2,128.9,128.2,127.8,123.0,109.2,81.4,60.1,36.8,34.5,26.9,22.4,13.9; HRMS(ESI)calcd for C 22 H 23 N 2 O 4 S[M+H] + m / z:411.1379,found411.1372;
[0051] 3-Butyl-6-(5-benzylsulfoxide)-1,3,4-oxadiazole-2-methoxy)-3-butylbenzofuranone-1(3H)-one ( 8c): colorless liquid, yield 53.8%; 1 H NMR (600MHz, CDCl 3 )δ7.41(d,J=2.4Hz,1H),7.39...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com