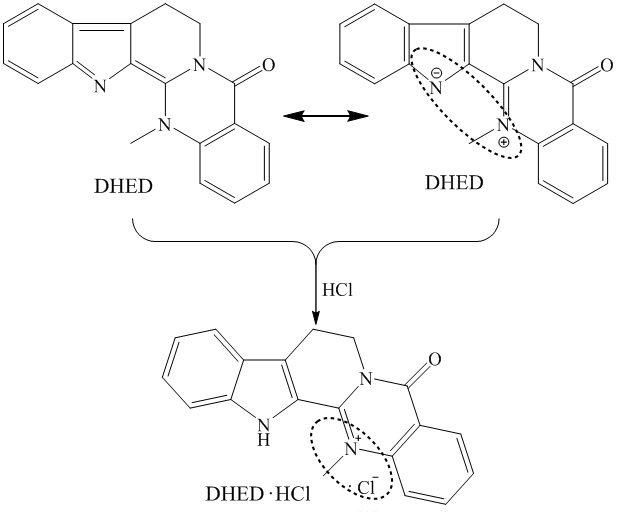
Chromatographic analysis method of dehydroevodiamine
A technique for dehydroevodiamine and chromatographic analysis, which is applied in the field of chromatographic analysis, can solve problems such as tailing, and achieves the effects of convenient operation, simple analysis conditions and simple preparation.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] A chromatographic analysis method for dehydroevodiamine in Evodia, the concrete steps are:
[0030] (1) Preparation of the reference substance solution: Precisely weigh 3.12 mg of the reference substance, dehydroevodiamine, and place it in a 10 mL volumetric flask, add anhydrous methanol to dissolve and dilute to the mark, and shake well to obtain the reference substance stock solution, which is stored in Refrigerator, dilute as needed before use;
[0031] (2) Evodia test sample solution: Take about 0.5 g of Evodia root powder, accurately weigh it, put it in a conical flask with stopper, add 25 mL of 70% ethanol precisely, weigh it, soak it for 1 h, sonicate it for 40 min, and let it Make up the lost weight by cooling, shake well and filter, take the filtrate, pass through a 0.22 μm filter membrane, and set aside;
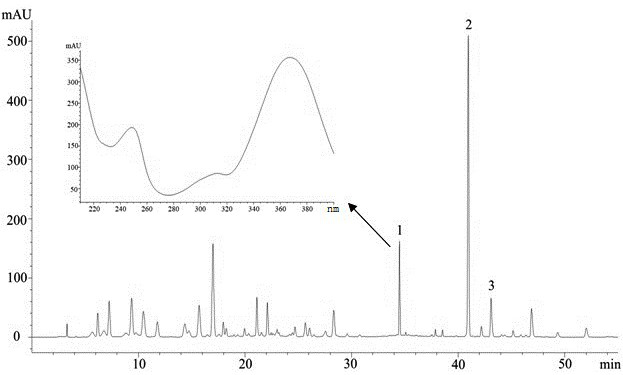
[0032] (3) Chromatographic conditions: DIKMA Platisil ODS column (4.6×250μm, 5μm); mobile phase: methanol (A)-water (B), both containing 0.1mol / L HCl; grad...
Embodiment 2
[0035] The chromatographic analysis method of dehydroevodiamine in compound berberine tablets, the concrete steps are:
[0036] (1) Preparation of the test product: Take 10 pieces of compound berberine tablets, remove the sugar coating, crush them in a mortar, mix evenly, weigh 0.5 g, accurately weigh, accurately add 25 ml of methanol, weigh the weight, ultrasonically Extraction for 30 min, let cool, make up the weight with methanol, take an appropriate amount of sample solution and pass through a 0.22 μm organic filter membrane to obtain;
[0037] (2) Chromatographic conditions: Shimadzu WondaCract ODS-2 (4.6×150mm, 5μm); mobile phase: acetonitrile (A)—0.1mol / L HCl (B); gradient elution: 0~6min, 43%~63% A; Flow rate: 1.0 mL / min; Injection volume: 5 μL; Column temperature: 30°C; Detection wavelength: 370 nm;
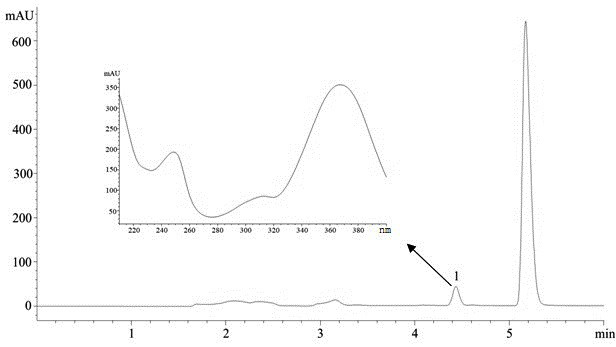
[0038] (3) Chromatographic separation efficiency: see the chromatogram image 3 , Dehydroevodiamine obtained a good chromatographic separation, and its chromatographic...
Embodiment 3
[0040] A chromatographic analysis method for dehydroevodiamine in rat plasma samples, the specific steps are:
[0041](1) Rats and methods of administration: SPF grade SD rats, male, body weight (200±20) g, 24 rats, provided by Hunan Slike Jingda Laboratory Animal Co., Ltd., license number SCXK (Xiang) 2016- 0002. The animals were reared at a temperature of 20-22°C, light / dark 12h / 12h, with free food and water, and the experiment was started after one week of adaptive rearing. Dehydroevodiamine was suspended in 0.5% CMC-Na and administered by gavage at a dose of 2.0 g / kg.
[0042] (2) Preparation of samples: Blank plasma sample: Take 50 μL of blank plasma sample, put it in a 1.5 mL centrifuge tube, add 1 mL of acetonitrile, vortex and mix for 2 min (deproteinized), and centrifuge at 4000 rpm for 10 min. The supernatant was taken, blown with nitrogen at 40°C, evaporated to dryness, reconstituted with 100 μL of 50% acetonitrile, vortexed for 2 min, and centrifuged at 14,000 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com