Antibody directional marking colloidal gold immune probe, preparation method thereof and lead ion test paper based on antibody directional marking
A technology of colloidal gold and lead ions, which is applied in antibody-directed labeling of colloidal gold immunoprobes and its preparation, and in the field of lead-ion test paper based on antibody-directed labeling, can solve the problems of uncontrollable antibody spatial orientation and low effective labeling of antibodies, and achieve Reduce detection cost, sensitive detection, low detection cost effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
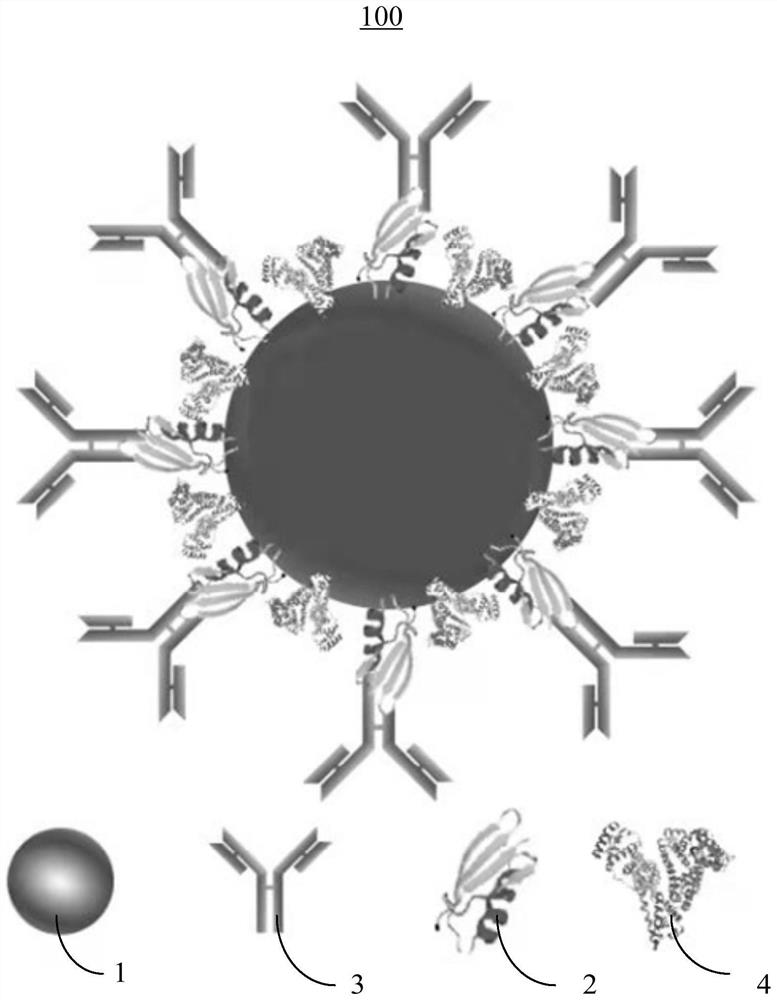
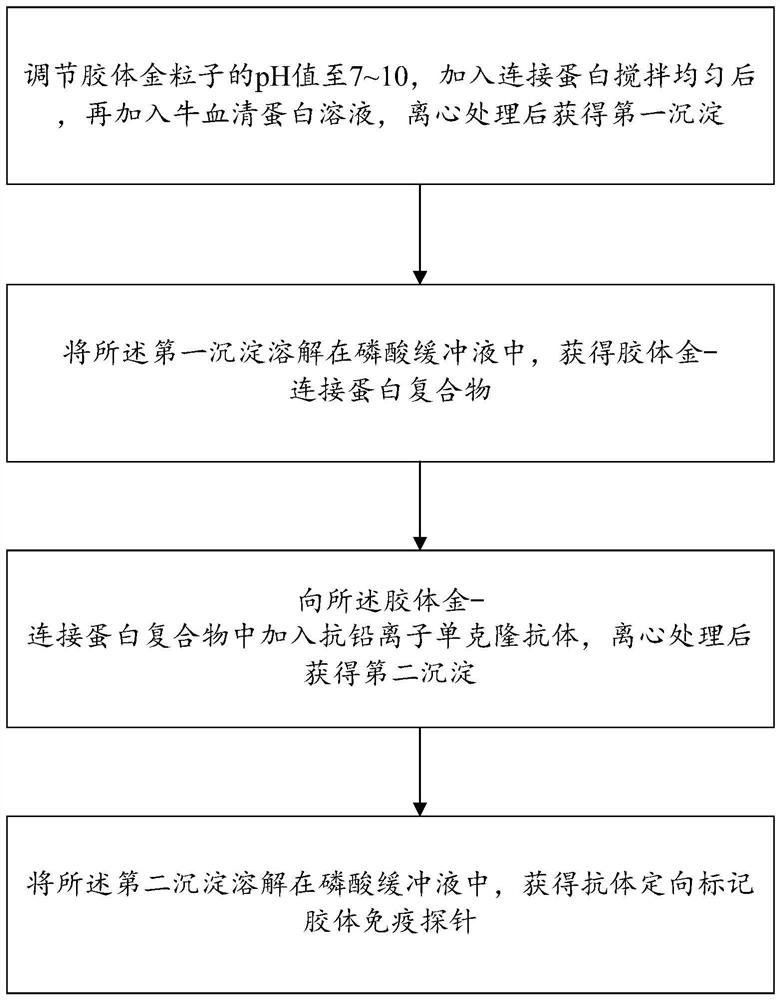
[0052] Also, see figure 2 , the present invention provides a method for preparing an antibody-directed labeled colloidal gold immunoprobe, and the antibody-directed labeled colloidal gold immunoprobe 100 prepared by the method for preparing an antibody-directed labeled colloidal gold immunoprobe is subjected to lead ion concentration The detection can ensure the full exposure of the antibody antigen binding site (Fab), improve the effective labeling of the antibody on the surface of colloidal gold, reduce the amount of the antibody, and reduce the detection cost, and the preparation method of the antibody-directed labeled colloidal gold immunoprobe Include the following steps:
[0053] Step S10, adjusting the pH of the colloidal gold particles 1 to 7-10, adding connexin 2 and stirring evenly, then adding bovine serum albumin 4 solution, and centrifuging to obtain a first precipitate.
[0054] In this embodiment, before preparing the first precipitation, the pH value of the c...
Embodiment 1
[0073] (1) Preparation of antibody-directed labeled colloidal gold immunoprobes
[0074] Add potassium carbonate solution dropwise to the colloidal gold particles, adjust the pH to 7.3, add recombinant protein G according to the mass ratio of colloidal gold particles and recombinant protein G to 100:18, react at 37°C for 30 min, and then add The bovine serum albumin solution with a concentration of 0.5% was reacted at 37° C. for 30 min, and then centrifuged at 9500 r / min for 13 min. After 7 times of centrifugation, the first precipitate was obtained.
[0075] The first precipitate was dissolved in a phosphate buffer with a concentration of 0.01 mol / L to obtain a colloidal gold-connexin complex.
[0076] According to the mass ratio of colloidal gold particles and anti-lead ion monoclonal antibody to 100:17.5, anti-lead ion monoclonal antibody was added to the colloidal gold-connexin complex, and the reaction was carried out at 37 °C for 120 min, and then at 8000 r / Centrifuge ...
Embodiment 2
[0085] (1) Preparation of antibody-directed labeled colloidal gold immunoprobes
[0086] Add potassium carbonate solution dropwise to the colloidal gold particles, adjust the pH value to 9.8, add recombinant protein A according to the mass ratio of colloidal gold particles and recombinant protein A to 100:3, react at 28 °C for 50 min, and then add The bovine serum albumin solution with a concentration of 0.55% was reacted at 28° C. for 50 min, and then centrifuged at 11,000 r / min for 12 min, and the first precipitate was obtained after centrifugation for 6 times.
[0087] The first precipitate was dissolved in a phosphate buffer with a concentration of 0.01 mol / L to obtain a colloidal gold-connexin complex.
[0088] According to the mass ratio of colloidal gold particles and anti-lead ion monoclonal antibody is 100:12.3, add anti-lead ion monoclonal antibody to the colloidal gold-connexin complex, react at 28°C for 150min, and then at 10000r / min Centrifuged for 8 min at 100 r...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com