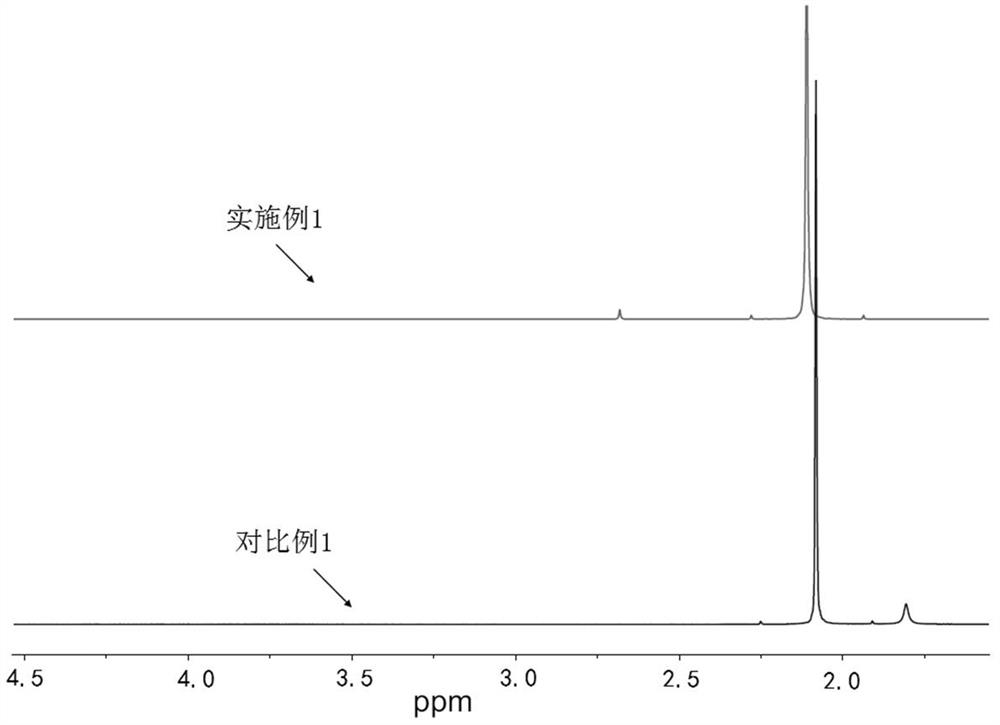
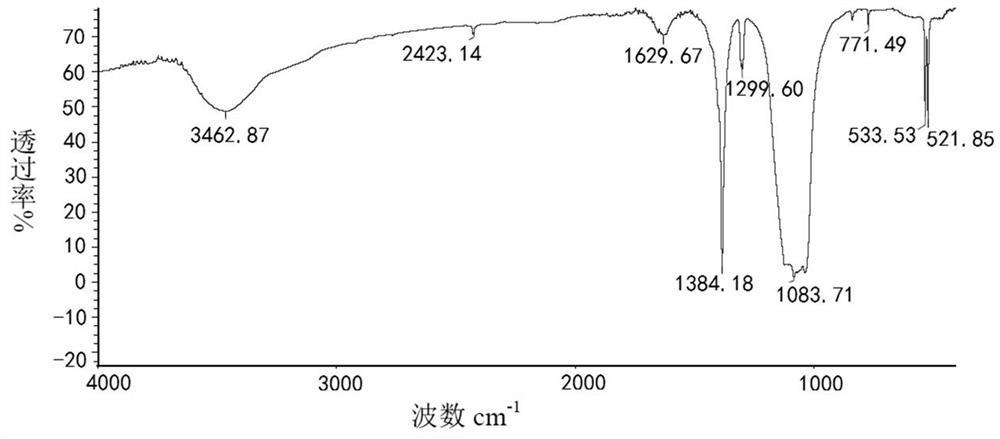
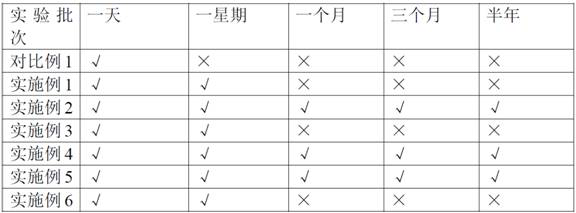
Preparation method of tetrakis (acetyl nitrile) silver tetrafluoroborate
A technology of silver tetrafluoroborate and acetonitrile is applied in the preparation of carboxylic acid nitrile, the preparation of organic compounds, chemical instruments and methods, etc., and can solve the problems of many impurities, high post-processing cost, and many steps.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0035] The invention provides a preparation method of tetrakis (acetonitrile) silver tetrafluoroborate, comprising the following steps:
[0036] Silver nitrate and acetonitrile are mixed and dissolved to prepare a first reaction solution;
[0037] The first reaction solution is mixed and reacted with ammonium fluoroborate to prepare the tetrakis(acetonitrile)silver tetrafluoroborate.
[0038] In one specific example, the ratio of the silver nitrate to acetonitrile is 50g:(300~550)mL. Specifically, the ratio (g:mL) of silver nitrate to acetonitrile includes but is not limited to: 50g:300mL, 50g:350mL, 50g:400mL, 50g:450mL, 50g:500mL, 50g:550mL. Further, the ratio of described silver nitrate and acetonitrile is 50g:(300~400)mL.
[0039] In one specific example, the molar ratio of the silver nitrate to the ammonium fluoroborate is 1:(1.2~1.4). Specifically, the molar ratio of silver nitrate to ammonium fluoroborate includes but is not limited to: 1:1.2, 1:1.25, 1:1.3, 1:1.35, ...
Embodiment 1
[0050] The present embodiment is a preparation method of tetrakis (acetonitrile) silver tetrafluoroborate, and the steps are as follows:
[0051] (1) Add 62.6g silver nitrate into a 500mL single-neck flask, add 300mL acetonitrile and stir to dissolve;
[0052] (2) Add ammonium fluoroborate (silver nitrate: ammonium fluoroborate molar ratio = 1:1.2) and continue to stir the reaction, the water bath is heated to 60 °C, and the reaction is performed for 4 hours;
[0053] (3) After the solution after the reaction is filtered twice with a filter membrane (pore size is 0.2 μm), the filtrate is rotary-evaporated at a temperature of 50 °C, and the filtrate is rotary-evaporated to about the theoretical product weight. In a viscous state, some silver nitrate remains;
[0054] (4) Dissolve the product of step (3) in a saturated solution of 300 mL of ammonium fluoroborate-acetonitrile, and a white precipitate (ammonium nitrate) is produced. Add 10 g of ammonium fluoroborate to the mixed ...
Embodiment 2
[0056] The present embodiment is a kind of preparation method of tetrakis (acetonitrile) silver tetrafluoroborate, and the main difference with embodiment 1 is: increase the feeding amount of acetonitrile and ammonium fluoroborate, wherein the feeding amount of acetonitrile is 400mL, silver nitrate: fluorine The molar ratio of ammonium borate=1:1.4; prolong the reaction time to 6h.
[0057] Specific steps are as follows:
[0058] (1) Add 62.6g silver nitrate into a 500mL single-neck flask, add 400mL acetonitrile and stir to dissolve;
[0059] (2) Add ammonium fluoroborate (silver nitrate: ammonium fluoroborate molar ratio = 1:1.4) and continue to stir the reaction, the water bath is heated to 60 ℃, and the reaction is performed for 6 hours;
[0060] (3) After the solution after the reaction is filtered twice with a filter membrane (pore size is 0.2 μm), the filtrate is rotary-evaporated at a rotary evaporation temperature of 50°C, and the filtrate is rotary-evaporated to abou...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pore size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com