Bile acid marker composition, application and serum detection kit
A bile acid and marker technology, applied in the fields of laboratory medicine, clinical chemistry, and clinical medicine, can solve the problems of poor patient compliance, poor accuracy of serum tumor markers, and low popularity of gastroscopic examination, and achieve simple combination and diagnostic sensitivity. Effectiveness with highly specific, noninvasive patient compliance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
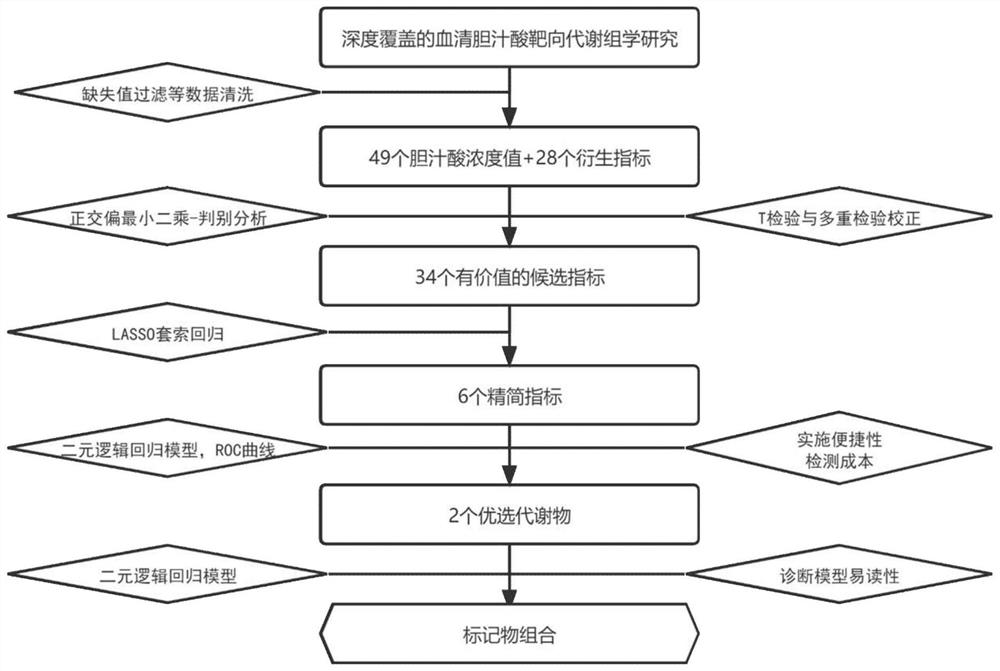
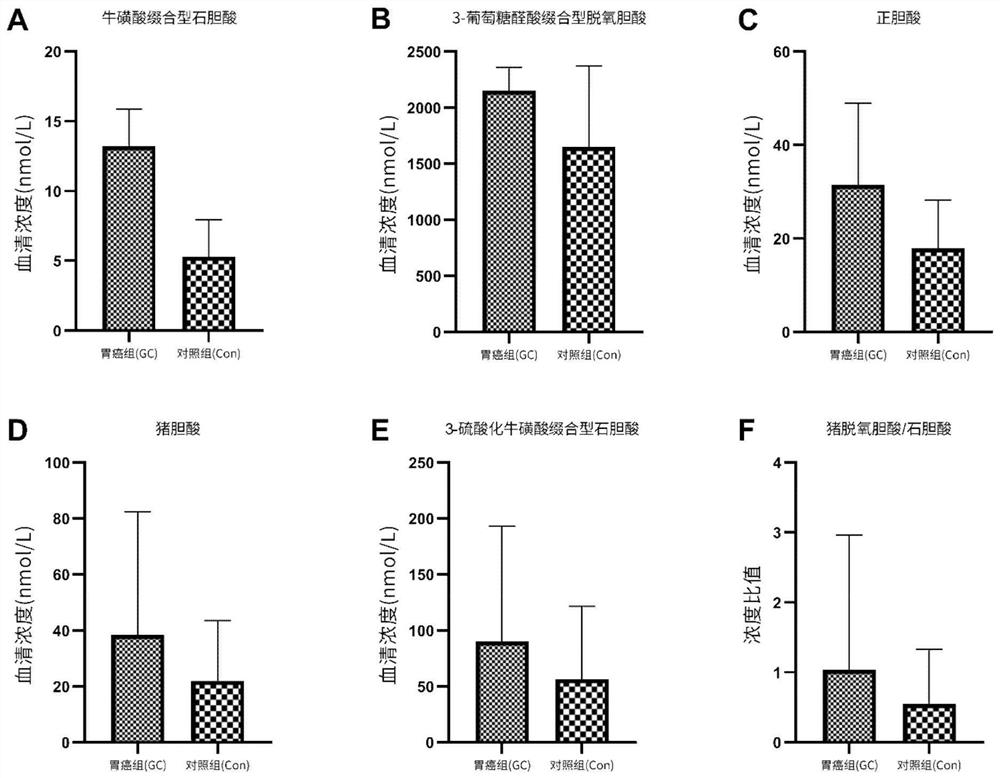
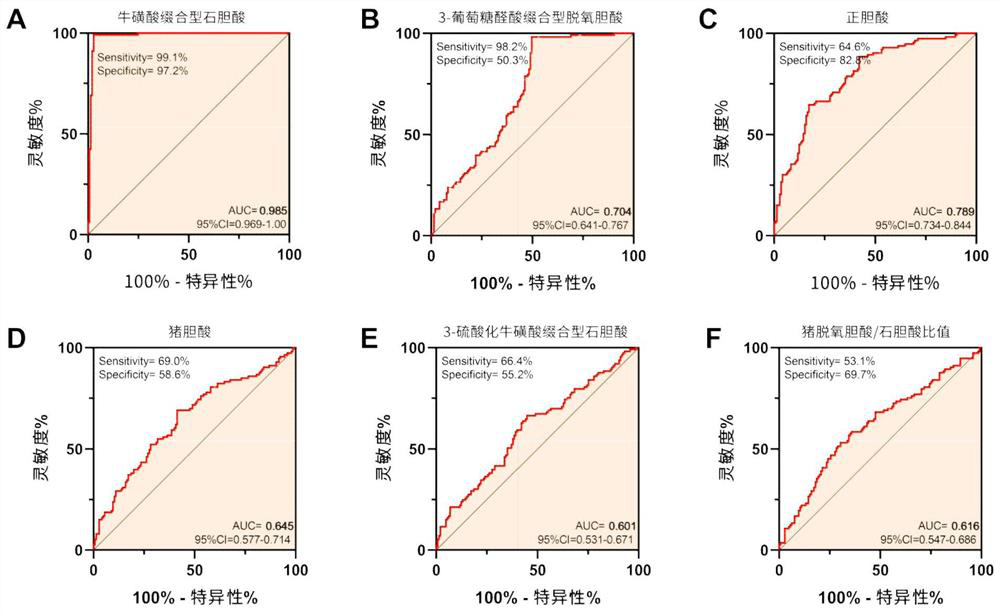
[0019] [Example 1] Auxiliary screening for gastric cancer based on the combination of two bile acid markers
[0020] 1. Clinical trial design: The research project has been approved by the Medical Ethics Association, and the subjects signed an informed consent form before being included in the clinical trial. The training set used to construct the gastric cancer screening and diagnosis model included 145 subjects in the healthy control group (age 60.31±12.15 years, 108 males and 37 females) and 113 subjects in the gastric cancer group (age 63.33±10.59 years old, There were 79 males and 34 females), of which 68 were advanced gastric cancer (cancer tissue infiltrated beyond the submucosa of the gastric wall and invaded the muscular layer of the gastric wall under the pathological section), and 45 were early gastric cancer (the cancer tissue had not invaded the gastric wall under the pathological section). Muscular layer, limited to gastric mucosa and submucosa). According to th...
Embodiment 2
[0037] [Example 2] Auxiliary screening for gastric cancer based on the combination of three bile acid markers
[0038] 1. Clinical trial design: same as Example 1 (ie, using the same subjects as Example 1).
[0039] 2. Subject serum collection: the same process and conditions as in Example 1.
[0040] 3. Pretreatment of the subject's serum to be tested: On the basis of Example 1, the extract added an internal standard substance deuterated Beta-murocholic acid-d5, and the concentration was 50ng / mL, and the rest were the same as in Example 1. This corresponding process and condition.
[0041] 4. Liquid chromatography tandem mass spectrometry detection: the same process and conditions as in Example 1.
[0042] 4.1 Liquid chromatography method: the same process and conditions as in Example 1.
[0043] 4.2 Mass spectrometry method: On the basis of Example 1, the detection of n-cholic acid was added, and the rest were the same as those of Example 1. The scanning ion pair of posi...
Embodiment 3
[0053] [Example 3] Auxiliary screening for gastric cancer based on the combination of four bile acid markers
[0054] 1. Clinical trial design: same as Example 1 (ie, using the same subjects as Example 1).
[0055] 2. Subject serum collection: the same process and conditions as in Example 1.
[0056] 3. Pretreatment of the subject's serum to be tested: On the basis of Example 1, the internal standard substances deuterated Beta-mouse cholic acid-d5 and deuterated cholic acid-d4 were added to the extract, and the concentration was 50ng / mL, and the rest were the same as Example 1 This corresponding process and conditions.
[0057] 4. Liquid chromatography tandem mass spectrometry detection: the same process and conditions as in Example 1.
[0058] 4.1 Liquid chromatography method: the same process and conditions as in Example 1.
[0059] 4.2 Mass spectrometry method: On the basis of Example 1, the detection of orthocholic acid and porcine cholic acid was added, and the rest we...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com