New application and preparation method of hyaluronic acid fragment
A technology of hyaluronic acid and a manufacturing method, which is applied in the field of biomedicine, can solve the problems of a stable manufacturing method of bioactive hyaluronic acid fragment B-HA, limited research on new clinical applications of manufacturing principles, etc., and achieves good tissue permeability and safety. The effect of good permeability and good tissue permeability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
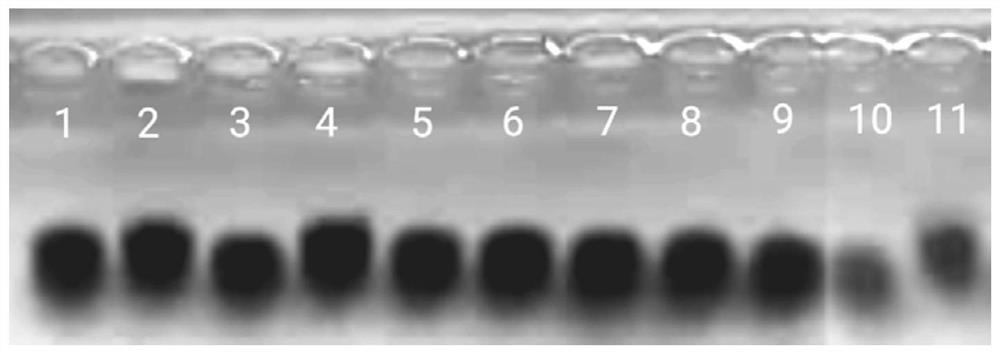
[0042] OBJECTIVE: To study the method of producing different low molecular weight hyaluronic acid fragments by fully enzymatically hydrolyzing high-molecular hyaluronic acid raw materials with different hyaluronidase enzymes or slightly excess.
[0043] method:
[0044] 1. Production of recombinant human hyaluronidase PH20
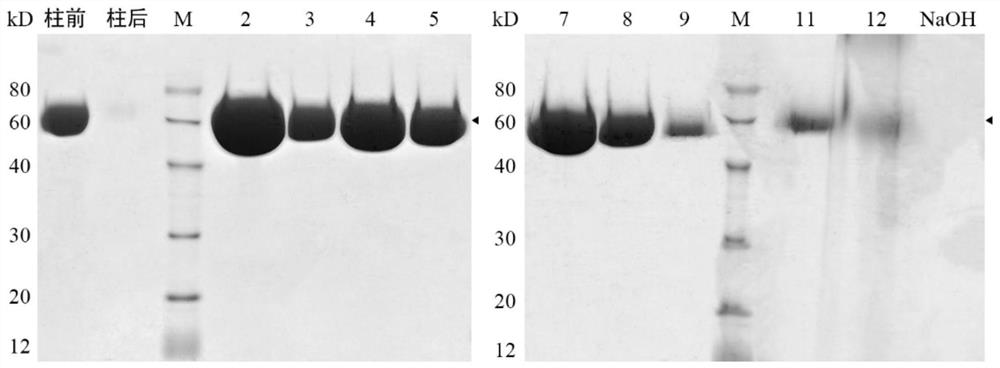
[0045] Based on the method described in reference [49], the cDNA of artificially synthesized recombinant human hyaluronidase PH20 was inserted into the GC-rich pMH3 vector to construct the pMH3-PH20 expression vector; then the pMH3-PH20 expression vector was transferred into CHO -S cell line, screened the CHO-S cell line with high expression of PH20, and carried out amplification and large-scale culture in the rapid flow animal cell reactor; the harvest liquid containing PH20 was filtered through a 0.22 μm filter membrane, and then passed through a QFF chromatography column, Phenyl HP hydrophobic chromatography column, CHT I type ceramic hydroxyapatite ch...
Embodiment 2
[0089] Objective: To study the rapid absorption pathway of hyaluronic acid fragment (hereinafter referred to as B-HA or HA35) with an average molecular weight of 35kDa (hereinafter referred to as B-HA or HA35) after subcutaneous and intravenous injection in mice after labeling with 125-I and 99mTc, and further use in vivo molecular imaging technology and human fresh extraction of human neutrophils and mononuclear cells (mainly lymphocytes and a small number of monocytes) to study their new therapeutic effects and new mechanisms of action.
[0090] Laboratory reagents, equipment, human and animal blood cell samples and laboratory animals:
[0091] 1. Experimental reagents: injection-grade hyaluronic acid raw materials with an average molecular weight of 1600kDa, common hyaluronic acid raw materials with an average molecular weight of 300kDa and hyaluronic acid fragments with an average molecular weight of 24kDa were purchased from Bloomage Freda Biomedical Co., Ltd., and the ave...
Embodiment 3
[0142] Objective: To use commercial bovine hyaluronidase PH20 injection and macromolecular hyaluronic acid injection to produce B-HA or HA35 with an average molecular weight of 35.4kDa in a short time to treat hypersensitivity of local large-area redness, swelling and hard pain caused by mosquito bites. Inflammation.
[0143] method:
[0144] Twelve cases of patients with hypersensitivity reaction to mosquito (mosquito or bumblebee) bites (with rapid localized large-area redness, swelling and pain in the bite area). Use the following 37 degrees for 20 minutes to mix the two injections and inject into the affected area outside the instructions, and then observe the change of the large area of red, swollen and hard pain in the bite area after treatment.
[0145] Before injection, bovine testicular hyaluronidase PH20 (1500u / piece, H31022111, Shanghai No. 1 Biochemical Pharmaceutical Factory) and sodium hyaluronate (hyaluronic acid) injection (trade name Speite) with an average...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com