Probiotic carrier, probiotic drops and preparation methods of probiotic carrier and probiotic drops
A technology of probiotics and carriers, applied in the field of probiotics, can solve the problems of reducing the circulation range of convenient products, reducing the decay rate of probiotics, and rapidly decreasing the activity of probiotics, and achieving good practical production feasibility and good food safety. , The effect of excellent complex dispersion performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
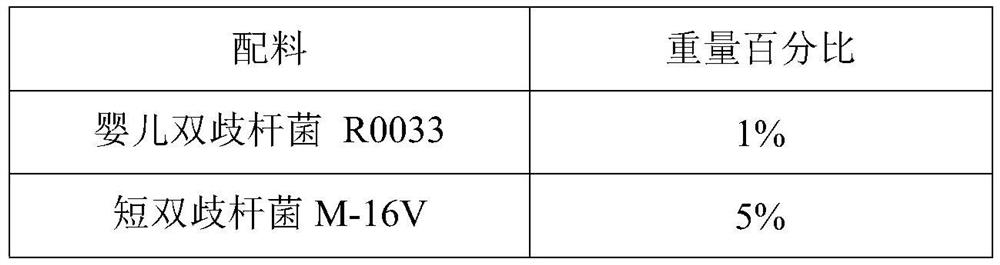
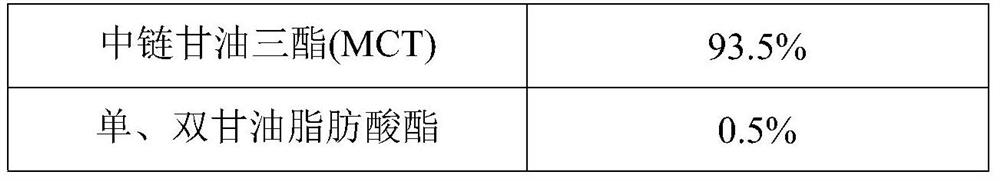
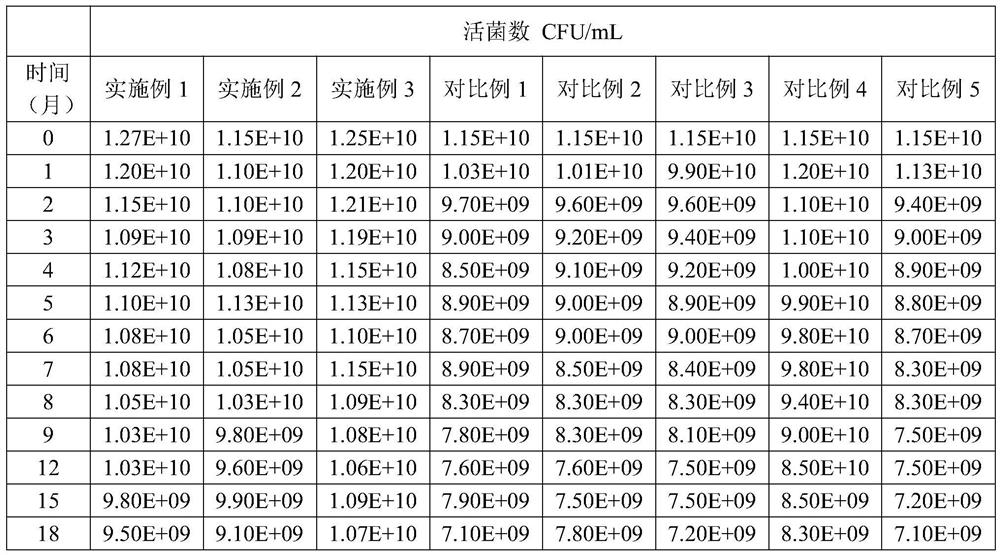
[0050] A probiotic bacteria drop is prepared from the formula in Table 1. The probiotic bacteria drop of Example 1 has good stability and excellent multi-dispersion performance, and can simultaneously improve infantile diarrhea and relieve infantile constipation.
[0051] The formula table of table 1 embodiment 1 probiotic bacteria drops
[0052]
[0053]
[0054] The probiotic bacteria drops of embodiment 1 are prepared according to the following method:
[0055] Bacteria thawing: Place Bifidobacterium breve M-16V bacterial powder and Bifidobacterium infantis R0033 bacterial powder at a temperature of 18°C and thaw for 48 hours. After thawing, dry the water droplets on the aluminum foil bag with an alcohol cloth and set aside.
[0056] Bifidobacterium breve M-16V, Bifidobacterium infantis R0033, mono- and diglyceride fatty acid esters and MCT oil were weighed according to the formula amount.
[0057] Pass Bifidobacterium breve M-16V bacterial powder and Bifidobacter...
Embodiment 2
[0062] A probiotic bacteria drop is prepared from the formula in Table 2. The probiotic bacteria drop of Example 2 has good stability and excellent complex dispersion performance, and can simultaneously improve infant diarrhea and relieve infant constipation.
[0063] The formula table of table 2 embodiment 2 probiotic bacteria drops
[0064] Ingredients weight percentage Bifidobacterium infantis R0033 5% Bifidobacterium breve M-16V 10% Medium Chain Triglycerides (MCTs) 83.5% Mono and diglycerol fatty acid esters 1.5%
[0065] The probiotic bacteria drops of embodiment 2 are prepared according to the following method:
[0066] Bacteria thawing: Place the Bifidobacterium breve M-16V bacterial powder and Bifidobacterium infantis R0033 bacterial powder at a temperature of 26°C and thaw for 12 hours. After thawing, dry the water droplets on the aluminum foil bag with an alcohol cloth and set aside.
[0067] Bifidobacterium breve M-16V, Bifi...
Embodiment 3
[0073] A probiotic bacteria drop is prepared from the formula in Table 3. The probiotic bacteria drop of Example 3 has good stability and excellent multi-dispersion performance, and can simultaneously improve infant diarrhea and relieve infant constipation.
[0074] The formula table of table 3 embodiment 3 probiotic bacteria drops
[0075] Ingredients weight percentage Bifidobacterium infantis R0033 3.0% Bifidobacterium breve M-16V 7.0% Medium Chain Triglycerides (MCTs) 89.0% Mono and diglycerol fatty acid esters 1.0%
[0076] The probiotic bacteria drops of embodiment 3 are prepared according to the following method:
[0077] Bacteria thawing: Place Bifidobacterium breve M-16V bacterial powder and Bifidobacterium infantis R0033 bacterial powder at a temperature of 22°C and thaw for 30 hours. After thawing, dry the water droplets on the aluminum foil bag with an alcohol cloth and set aside.
[0078] Bifidobacterium breve M-16V, Bifidob...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size (mesh) | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap