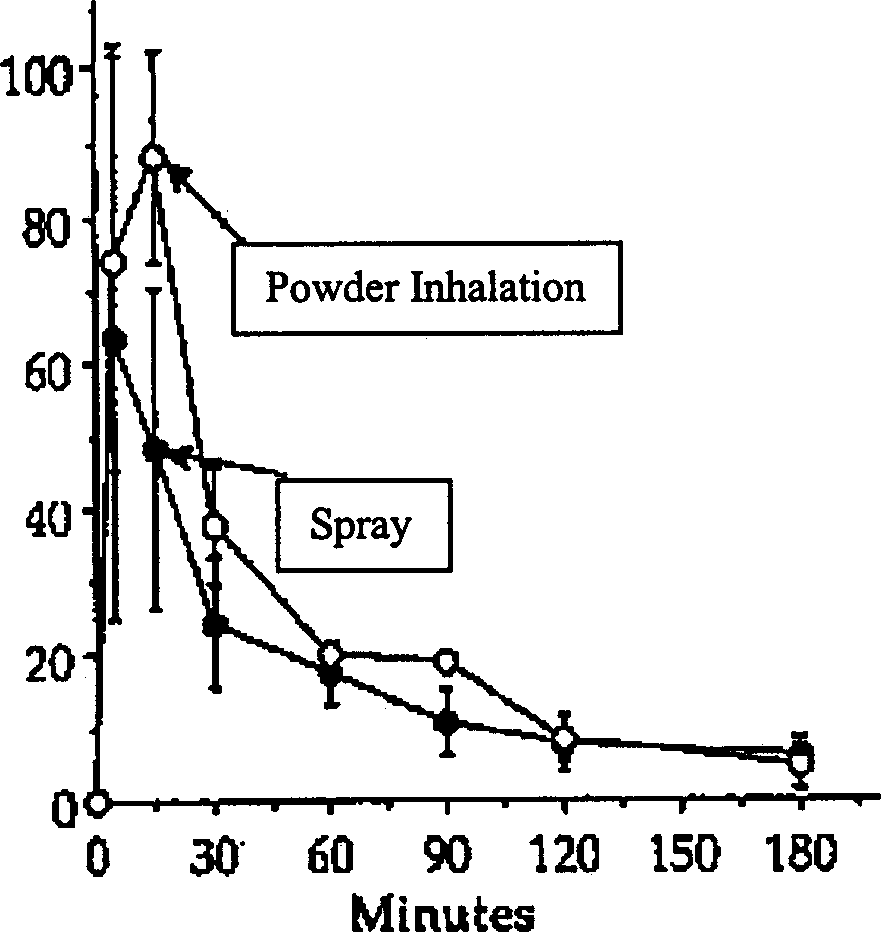
Inhalant of Shumaputan dry-powder and its preparation method
A technology of dry powder inhaler and sumatriptan, which is applied in the fields of pharmaceutical formulation, powder delivery, and medical preparations containing active ingredients, etc., which can solve the problems of expensive drug waste, low drug stability, and inconvenient carrying and use.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
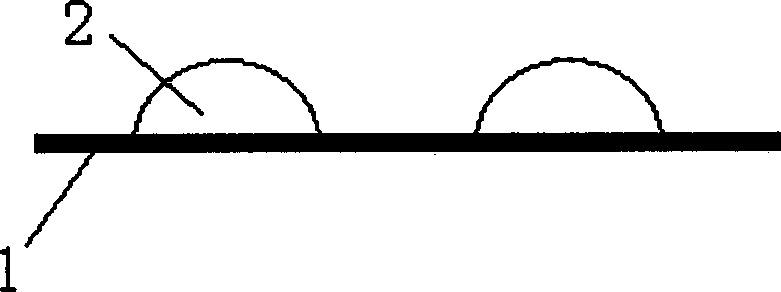
[0051] Separately pulverize 5 grams of sumatriptan, 2 grams of sodium carboxymethylcellulose, 3 grams of microcrystalline cellulose (specific name), 10 grams of diluent (lactose) and 0.1 grams of surfactant (poloxamer) Finally, pass through a 200-mesh sieve, dissolve and spray-dry at 80°C, select agglomerates that meet the particle size requirements, and quantitatively distribute them in such as figure 2 Inside the blister 1 of the double-dose blister shown, the preparation according to the invention is obtained.
Embodiment 2
[0053] 5 grams of sumatriptan, 2 grams of gel adhesive (sodium alginate), 2 grams of gel adhesive (hydroxypropyl cellulose), 2 grams of water-insoluble adhesive (chitin), 10 grams Diluents (5 grams of β-cyclodextrin, 5 grams of mannitol) were pulverized respectively, passed through a 200 mesh sieve, granulated with 1% polysorbate 80 aqueous solution as a binding agent, dried at 60 ° C, and selected to meet the particle size requirements. Agglomerates, add 5 grams of carrier (lactose with an angle of repose less than 40°), quantitatively dispense in such as figure 2 Inside the blister 1 of the double-dose blister shown, the preparation according to the invention is obtained.
Embodiment 3
[0055] 5 grams of sumatriptan, 2 grams of gel adhesive (hydroxypropylmethylcellulose), 1 gram of gel adhesive (starch), 2 grams of water-insoluble adhesive (methylcellulose), After 5 grams of diluent (lactose) is pulverized respectively, cross 200 mesh sieves, use 75% ethanol-water (v / v) solution as adhesive granulation, dry at 60 ℃, select the agglomerate that meets particle size requirements, add 10 grams of carrier (pregelatinized starch with an angle of repose less than 40°), quantitatively dispensed in such as figure 2 Inside the blister 1 of the double-dose blister shown, the preparation according to the invention is obtained.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com