Detection kit for autosomal dominant inheritance polycystic kidney disease
A detection kit and autosomal technology, applied in the field of medical molecular biology detection, can solve the problems of reduced detection specificity, complex structure, lack of uniformity and repeatability, etc., and achieve the effect of preventing errors and increasing the number of primers
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0054] 1. Preparation of each solution of the primer mix
[0055] (1) 0.8mol / L Tris solution
[0056] Add 96.9 g of tris to 800 ml of deionized ultrapure water, adjust the pH to 8.9 with concentrated hydrochloric acid, and dilute to 1 L with deionized ultrapure water.
[0057] (2) 1.7mol / L potassium acetate solution
[0058] Add 166.8 g of potassium acetate to 800 ml of deionized ultrapure water, and after fully dissolving, dilute to 1 L with deionized ultrapure water.
[0059] (3) Dimethyl sulfoxide solution
[0060] The dimethyl sulfoxide solution with a concentration greater than 99% is dispensed in 10ml / bottle for use.
[0061] (4) 20% Octylphenoxypolyethoxyethanol
[0062] Take 1.88 ml of commercially available octylphenoxypolyethoxyethanol (Shanghai Huamei Biotechnology Co., Ltd.) with a concentration of 106.5% (w / v), and dilute to 10 ml with deionized ultrapure water.
[0063] (5) Deoxynucleoside triphosphate solution
[0064] A commercial deoxynucleoside triphosp...
Embodiment 1
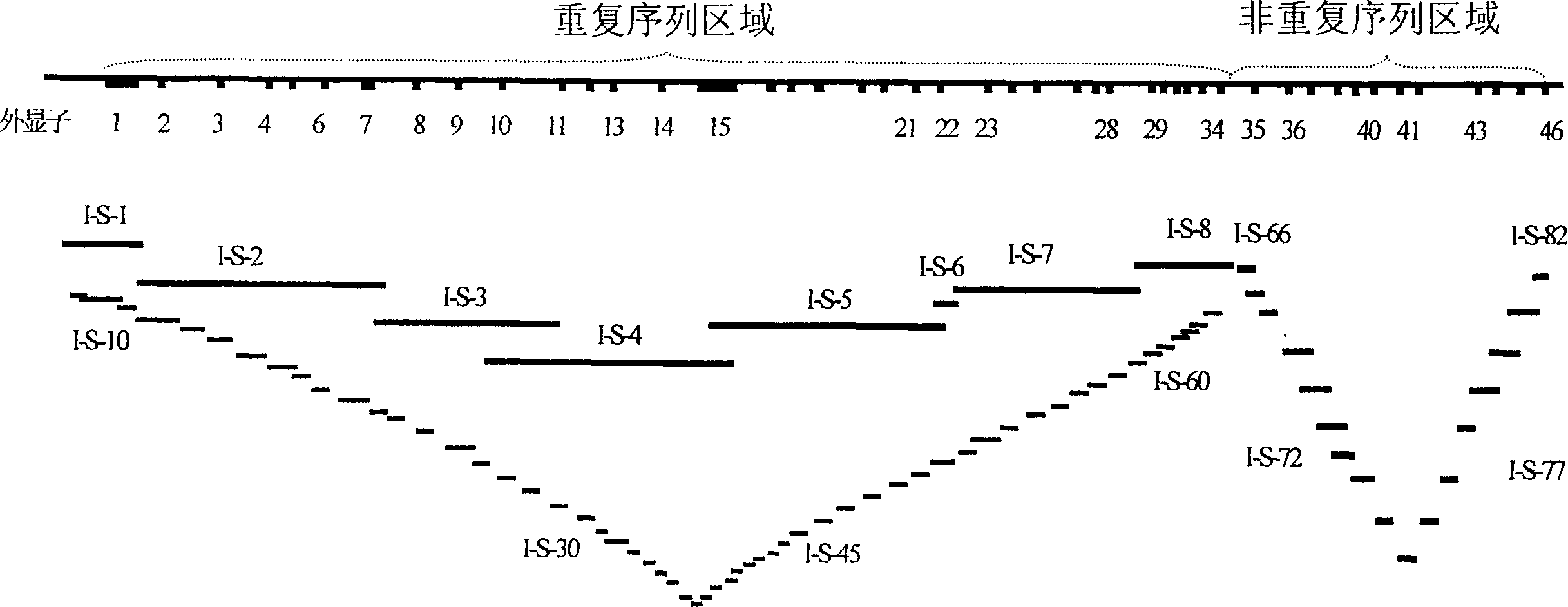
[0074] Example 1: Preparation of Primer Mix I-S-1
[0075] Take 20 μl of 0.8 mol / L tris hydroxymethyl aminomethane solution, 20 μl of 1.7 mol / L potassium acetate solution, 20 μl of dimethyl sulfoxide solution, 2 μl of 20% octylphenoxy polyethoxyethanol, 10 mmol / L deoxygenated nucleus Glycoside triphosphate solution 24μl, 250mmol / L magnesium acetate solution 2μl, enzyme activity 4U / μl thermostable deoxyribonucleotide polymerase 20μl, 50μmol / LI-S-1 forward and reverse primers 8μl each, mix well , dilute to 200 μl with sterilized deionized ultrapure water, put in a 0.5ml thin-walled tube and store at -20°C for later use. At this time, the final concentrations are: Tris 80mmol / L, potassium acetate 170mmol / L, dimethyl sulfoxide 10%, octylphenoxypolyethoxyethanol 0.2%, deoxynucleoside triphosphate 1200μmol / L , magnesium acetate 2.5mmol / L, thermostable deoxyribonucleotide polymerase 80U, forward and reverse primers 2μmol / L each.
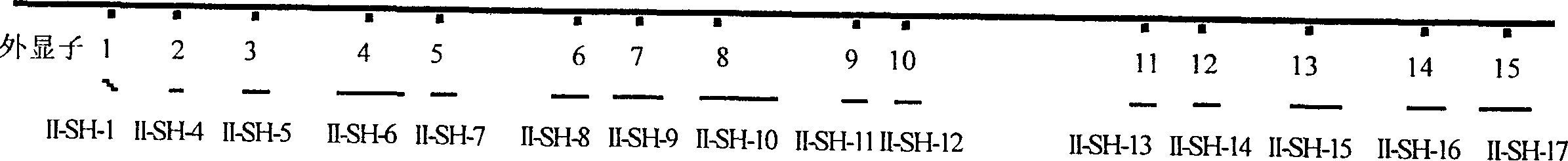
[0076] The preparation method of primer mixture I-H...
Embodiment 2
[0077] Example 2: Preparation of Primer Mix I-S-2
[0078] Take 10 μl of 10 mmol / L deoxynucleoside triphosphate solution, the enzymatic activity is 4 U / μl thermostable deoxynucleotide polymerase 4 μl The amounts of other components are the same as in Example 1. After thorough mixing, dilute to 200 μl with sterilized deionized ultrapure water, put in a 0.5ml thin-walled tube and store at -20°C for later use.
[0079] The preparation method of primer mixtures I-S-3 to I-S-8 and I-H-2 and I-H-8 is the same as that in Example 2.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com