Method of synthesizing metal-tetracyano-p-benzoquinone dimethane ester derivative
A technology of benzoquinodimethane and its synthesis method, which is applied in the field of synthesis of metal-tetracyano-p-benzoquinone dimethane ester derivatives, can solve problems such as low solubility, difficult spin coating molding, hindering the application of compounds, etc., and achieves The effect of high yield and easy control of reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Concrete synthetic steps are as follows:
[0024] Weigh raw materials according to the following weight percentage:
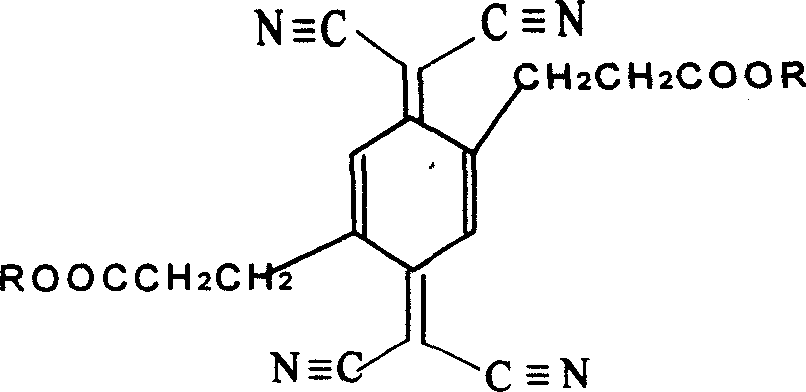
[0025] TCNQ ester derivatives choose 2,5-dipropionate methyl ester-7,7,8,8-tetracyanoquinone dimethane:
[0026] (TCNQ(CH 2 CH 2 COOCH 3 ) 2 ): 0.5%
[0027] NaI: 0.5%
[0028] CuI: 0.5%
[0029] Acetonitrile: 98.5%
[0030] At 85°C, dissolve methyl 2,5-dipropionate-7,7,8,8-tetracyanoquinodimethane in acetonitrile, and pass nitrogen protection;
[0031] Add NaI, stir to make NaI fully dissolve, add cuprous iodide (CuI), and react for 2 hours;
[0032] After the reaction is complete, the resulting crude product is filtered out, purified, and dried to obtain copper 2,5-dipropionate methyl ester-7,7,8,8-tetracyanoquinone dimethane derivatives Electron transfer complex:
[0033] Cu(TCNQ(CH 2 CH 2 COOCH 3 ) 2 )
[0034] Yield: 89%, melting point: 278°C.
[0035] The characteristic absorption peak of infrared spectrum is: 2960cm -1 , 2193...
Embodiment 2
[0038] Weigh the synthetic raw materials in the following percentages by weight:
[0039] TCNQ ester derivatives choose 2,5-dipropionate methyl ester-7,7,8,8-tetracyanoquinone dimethane:
[0040] (TCNQ(CH 2 CH 2 COOCH 3 ) 2 ): 0.6%
[0041] AgI: 0.6%
[0042] NaI: 0.6%
[0043] Acetonitrile: 98.2%
[0044] At 70°C, dissolve methyl 2,5-dipropionate-7,7,8,8-tetracyanoquinodimethane in acetonitrile, and pass through nitrogen protection;
[0045] Add NaI, stir to make NaI fully dissolve, add silver iodide (AgI), and react for 1.5 hours;
[0046] After the reaction is complete, the resulting crude product is filtered out, purified, and dried to obtain silver 2,5-dipropionate methyl ester-7,7,8,8-tetracyanoquinone dimethane derivatives Electron transfer complex:
[0047] Ag(TCNQ(CH 2 CH 2 COOCH 3 ) 2 )
[0048] Yield: 88%. The melting point is 278°C.
[0049] The characteristic absorption peak of infrared spectrum is: 2960cm -1 , 2193cm -1 , 1735cm -1 , 1637c...
Embodiment 3
[0052] Concrete synthetic steps are as follows:
[0053] Weigh the synthetic raw materials in the following percentages by weight:
[0054] TCNQ ester derivatives choose 2,5-ethyl dipropionate-7,7,8,8-tetracyanoquinone dimethane:
[0055] (TCNQ(CH 2 CH 2 COOCH 2 CH 3 ) 2 ):2%
[0056] CuI: 2%
[0057] NaI: 2%
[0058] Acetonitrile: 94%
[0059] Dissolve ethyl 2,5-dipropionate-7,7,8,8-tetracyanoquinodimethane in acetonitrile at 70°C, and pass through nitrogen protection;
[0060] Add NaI, stir to make NaI fully dissolve, add cuprous iodide (CuI), and react for 1.5 hours;
[0061] After the reaction is complete, the resulting crude product is filtered out, purified, and dried to obtain copper 2,5-dipropionate-7,7,8,8-tetracyanoquinone dimethane derivatives Electron transfer complex:
[0062] Cu(TCNQ(CH 2 CH 2 COOCH 2 CH 3 ) 2 ).
[0063] Yield: 89%, melting point: 250°C.
[0064] The characteristic absorption peak of the infrared spectrum is: 2955cm -1 , 21...
PUM
| Property | Measurement | Unit |
|---|---|---|
| solubility (mass) | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com