Eye gel for atropine sulfate
A technology of atropine sulfate and ophthalmic gel, which is applied in the fields of pharmaceutical formulations, sensory diseases, active ingredients of heterocyclic compounds, etc. It can solve problems such as short residence time, eye drops are easily diluted by tears, and oral drugs are difficult to pass through the blood-ocular barrier. , to achieve long residence time, avoid other side effects, and avoid gastrointestinal irritation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0008] Prescription 1:
[0010] Hypromellose 4.0g
[0011] Borax 1.0 g
[0012] Boric acid 0.5g
[0013] Chlorinated benzalkonium 0.01 g
[0014] Water for injection made 100g
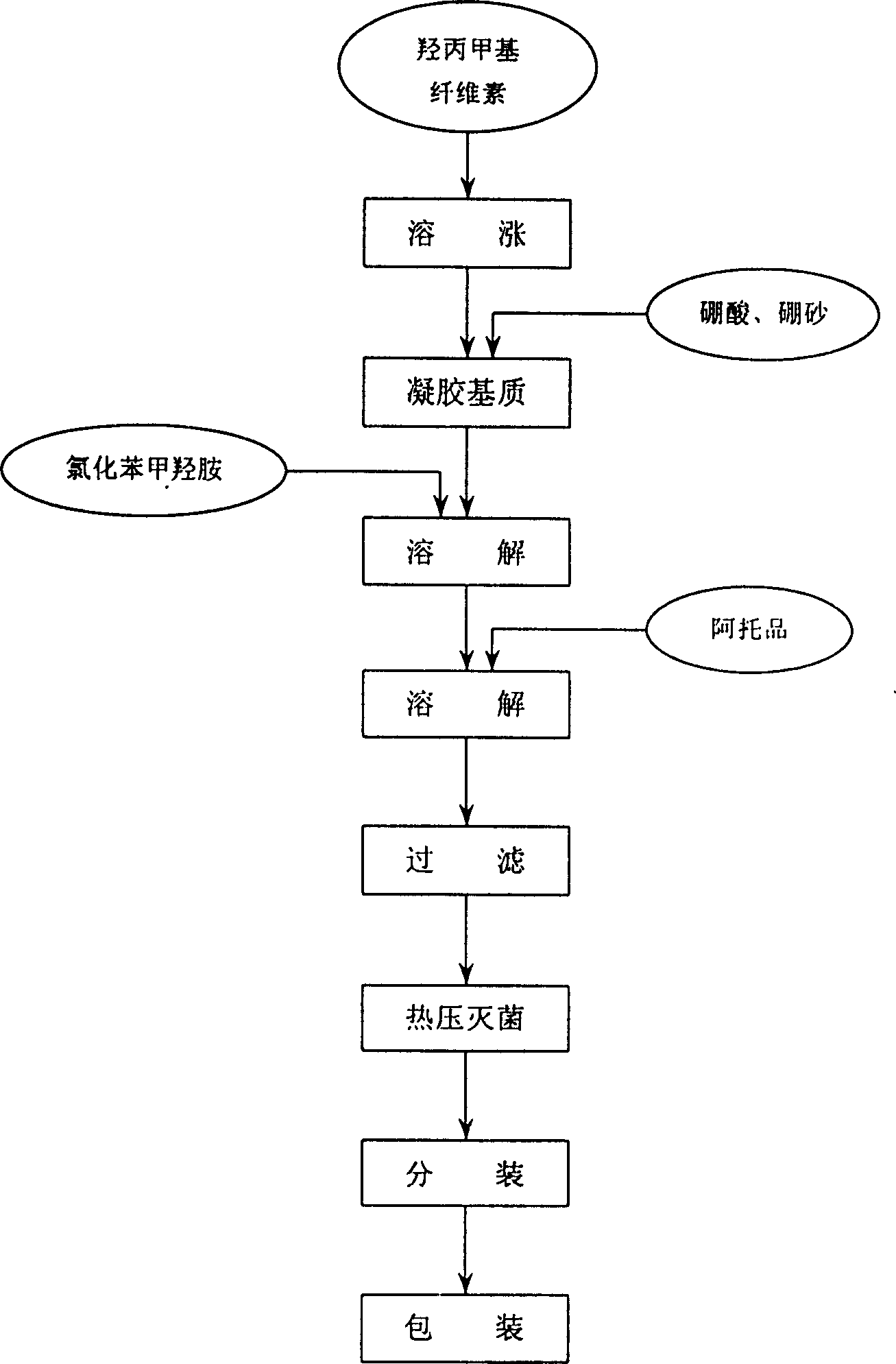
[0015] Operation process:
[0016] ①. Take the prescribed amount of hypromellose and swell with an appropriate amount of water for injection, overnight.
[0017] ②. Take the prescribed amount of borax and boric acid and add it, stir evenly, and heat to form a transparent gel matrix;
[0018] ③. Add benzalkonium chloride and stir to dissolve;
[0019] ④. Add atropine sulfate, add water to the full amount, and stir well;
[0020] ⑤. Filter through a 220-mesh sieve cloth, autoclave, and pack.
[0021] Prescription 2:
[0022] Atropine sulfate 0.1-5.0g
[0023] Hypromellose 0.1-20.0g
[0024] Borax 0.1-5.0g
[0025] Boric acid 0.1-2.5 grams
[0026] Chlorinated benzalkonamine 0.01-0.5 g
[0027] water for injection made 100g
[0028] Operation process:
[002...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com