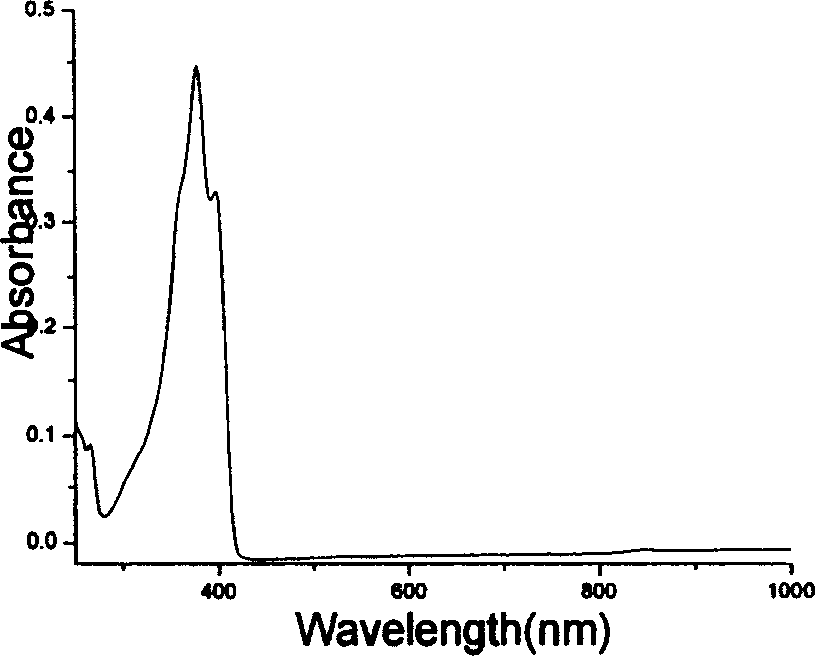
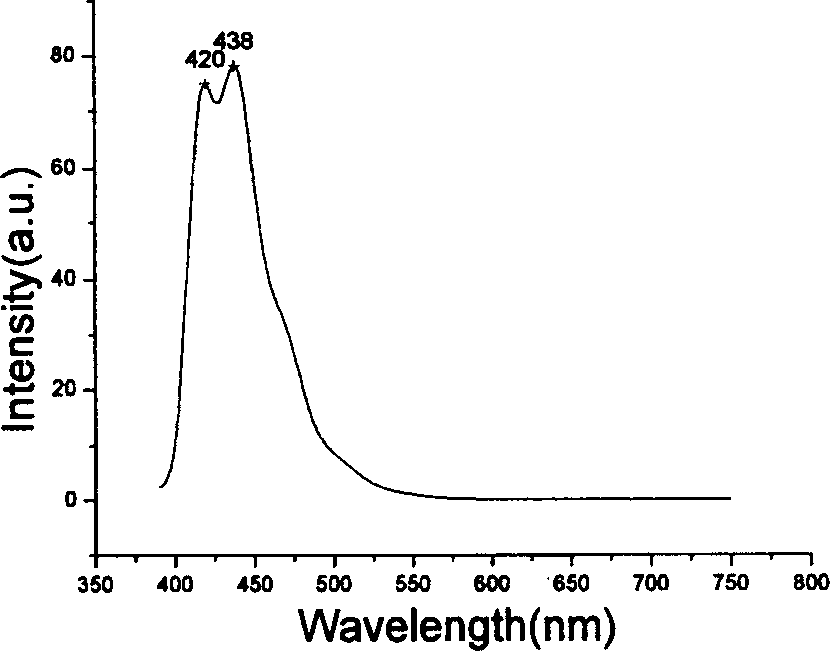
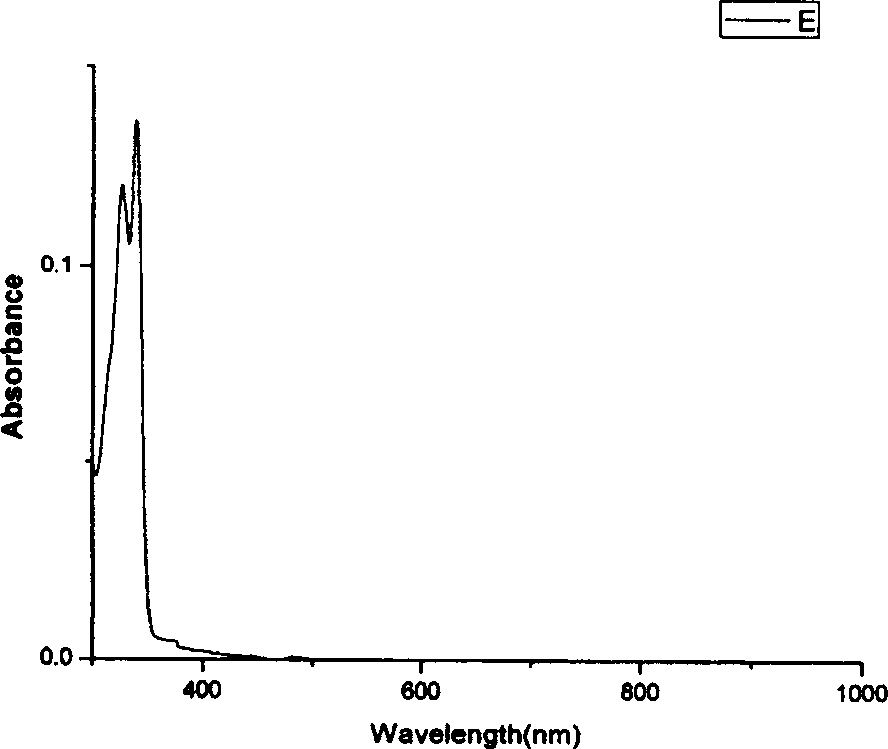
Fluorene derivatives and their application
A compound and group technology, applied in the field of novel fluorene derivatives, can solve the problems of limited application, weak two-photon absorption, limited application by thermal stability and photochemical stability, and achieve easy use and good solubility. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Synthesis of 1,1'-(9,9-bis(2-ethylhexyl)-2,7-fluorenyl-2,1-divinyl)bis(4-methoxy)benzene
[0031] Step 1
[0032] Synthesis of 9,9-bis(2-ethylhexyl)fluorene
[0033] Fluorene (34 g, 0.205 mol), potassium hydroxide (56 g, 1.0 mol), and potassium iodide (3.4 g, 0.02 mol) were added to 150 ml of DMSO, stirred to dissolve, and then (2-ethylhexyl) bromide was added dropwise (96ml, 103.5g, 0.536mol), the temperature was raised to 25°C and stirred for 24 hours. After the reaction, the mixture was poured into water, separated, washed, dried, concentrated, and then distilled under reduced pressure to obtain a crude product. Further purification by column chromatography on silica gel gave 9,9-bis(2-ethylhexyl)fluorene, 74.4 g, with a yield of 93%.
[0034] Step two:
[0035] Synthesis of 2,7-dibromo-9,9-bis(2-ethylhexyl)fluorene
[0036] Add 9,9-bis(2-ethylhexyl)fluorene (18.35 g, 0.047 mol) and iodine (0.12 g, 0.47 mmol) prepared in the above step into 170 ml of dichloro...
Embodiment 2
[0041] Synthesis of 1,1'-(9,9-diethyl-2,7-fluorenyl-2,1-divinyl)bis(4-methoxy)benzene
[0042] Step 1:
[0043] Synthesis of 9,9-diethylfluorene
[0044]The step is the same as step 1 in Example 1, except that (2-ethylhexyl) bromine is changed to bromoethane (40ml, 58.4 grams, 0.536mol) to obtain 9,9-diethylfluorene, 43.26 grams, product The rate is 95%.
[0045] Step two:
[0046] Synthesis of 2,7-dibromo-9,9-diethylfluorene
[0047] The step is the same as step 2 in Example 1, except that 9,9-bis(2-ethylhexyl)fluorene is changed into 9,9-diethylfluorene (10.44 grams, 0.047mol) to obtain 2,7-bis Bromo-9,9-diethylfluorene, 15.2 g, 85% yield.
[0048] Step three:
[0049] Synthesis of 1,1'-(9,9-diethyl-2,7-fluorenyl-2,1-divinyl)bis(4-methoxy)benzene
[0050] The steps are the same as step 3 in Example 1, except that 2,7-dibromo-9,9-bis(2-ethylhexyl)fluorene is changed to 2,7-dibromo-9,9-diethylfluorene (6.27 g, 16.5 mmol) to give 1,1'-(9,9-diethyl-2,7-fluorenyl-2,1-di...
Embodiment 3
[0052] Synthesis of 1,1'-(9,9-bis(dodecyl)-2,7-fluorenyl-2,1-divinyl)bis(4-methoxy)benzene
[0053] Step 1:
[0054] Synthesis of 9,9-Di(dodecyl)fluorene
[0055] Step is the same as step 1 in example 1, and difference is that (2-ethylhexyl) bromide is changed into dodecyl bromide (130ml, 133.6 grams, 0.536mol), obtains 9,9-bis(dodecyl) Fluorene, 104 g, yield 90%.
[0056] Step two:
[0057] Synthesis of 2,7-dibromo-9,9-di(dodecyl)fluorene
[0058] The step is the same as step 2 in Example 1, except that 9,9-bis(2-ethylhexyl)fluorene is changed into 9,9-bis(dodecyl)fluorene (23.62 grams, 0.047mol) to obtain 2 , 7-dibromo-9,9-bis(dodecyl)fluorene, 25.46 g, yield 82%.
[0059] Step three:
[0060] Synthesis of 1,1'-(9,9-bis(dodecyl)-2,7-fluorenyl-2,1-divinyl)bis(4-methoxy)benzene
[0061] The step is the same as step 3 in Example 1, except that 2,7-dibromo-9,9-bis(2-ethylhexyl)fluorene is changed to 2,7-dibromo-9,9-bis(dodecyl) Alkyl)fluorene (10.9 g, 16.5 mmol) to giv...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com