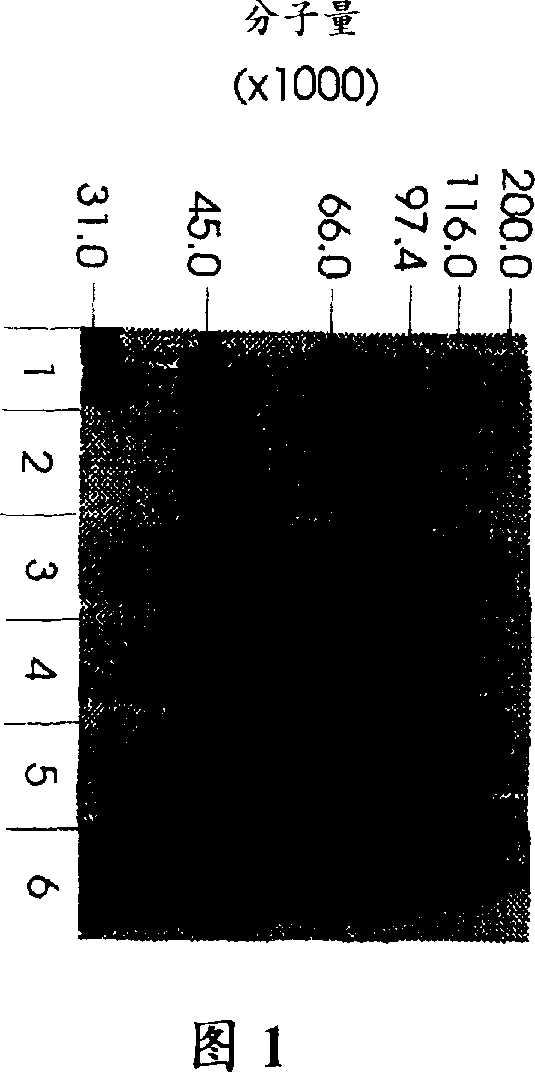
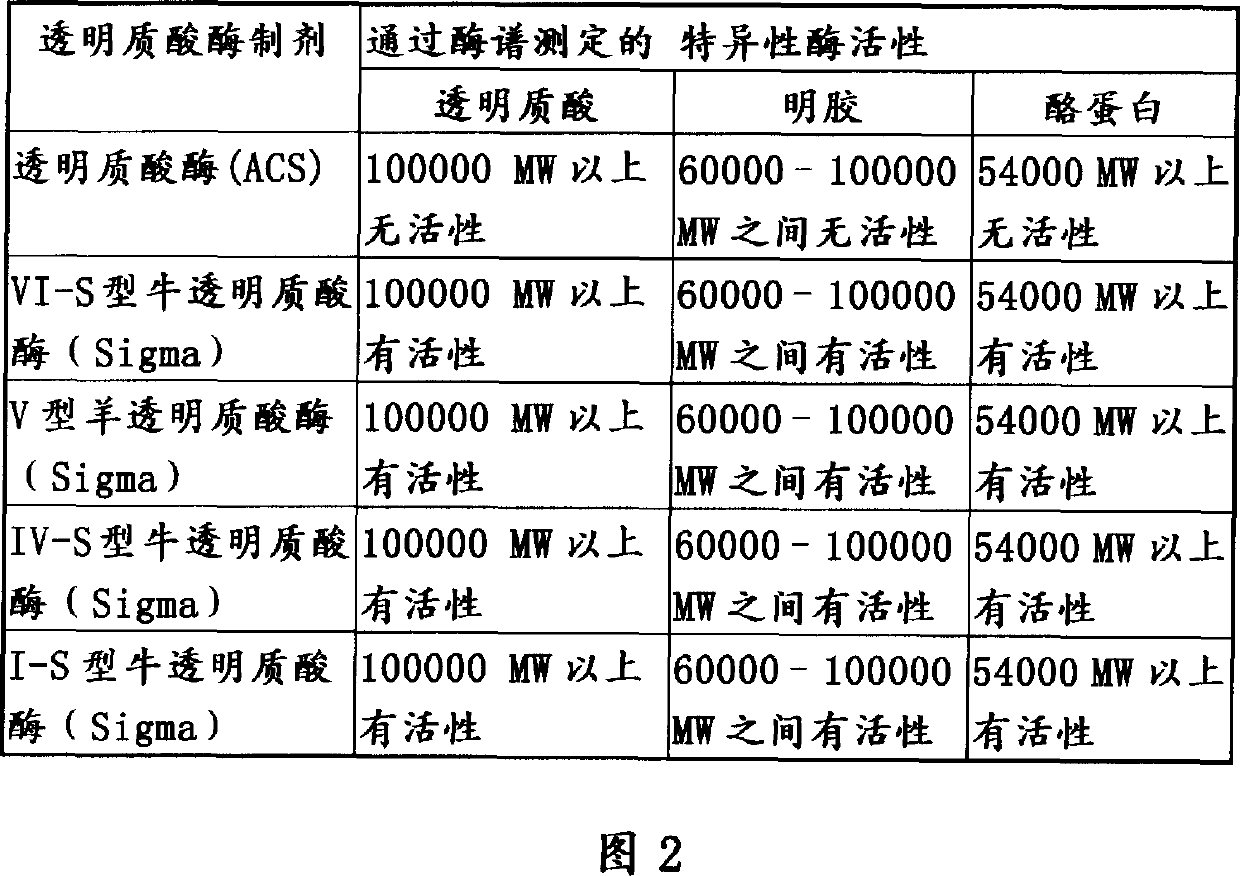
Use of hyaluronidase in the manufacture of an ophthalmic preparation for liquefying vitreous humor in the treatment of eye disorders
A hyaluronidase, vitreous humor technology, applied in metabolic diseases, sensory diseases, peptide/protein components, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0084] 52 healthy New Zealand Cross Variety rabbits (26 male rabbits, 26 female rabbits) weighing 1.5-2.5 kg were marked with identification marks and kept in hanging cages respectively. Rabbits were provided with a daily basal amount of commercial rabbit chow bolus and ad libitum access to tap water.
[0085] Rabbits were divided into 13 groups of 4 (2 males, 2 females). In each group, 2 rabbits (1 male, 1 female) were selected for fundus photography and fluorescein angiography pretreatment.
[0086] Fundus photography was carried out as follows: rabbits were fixed, and KOWA with Kodak Gold 200 ASA film was used The RC-3 fundus camera takes pictures of the optic nerve and retinal arch.
[0087] Fluorescein angiography involves injecting 1.5 ml of 2% sterile fluorescein solution via the marginal ear vein. About 30 seconds after injection, fluorescein develops in the optic nerve, retinal blood vessels and fundus.
[0088] The next day, each rabbit was anesthetized with an...
Embodiment 2
[0106] In this example, 12 healthy new acyl hybrid rabbits were marked with identification marks and kept in hanging cages. Rabbits were provided with a daily basal amount of commercial rabbit chow bolus and ad libitum access to tap water.
[0107] Rabbits were randomly divided into 4 treatment groups with 3 rabbits in each group.
[0108] First, the pupils of each rabbit's eyes were dilated with 1-2 drops of 10% tropicamide, followed by visual inspection, indirect ophthalmoscopy with a 20 diopter lens, and slit-lamp examination of the anterior chamber of the eye.
[0109] After the initial examination of the eyes of the rabbits, a 100:1 or 10:1 blood injection was injected into the vitreous of each eye of each rabbit.
[0110] On day 2, BSS or hyaluronidase ACS was injected into the vitreous of the right eye of each treatment group rabbit according to the following treatment schedule, where each rabbit received one injection: Group # Treatment
[0111] ...
Embodiment 3
[0120] The main objective of this study was to determine whether a balanced salt solution containing highly purified hyaluronidase extracted from bovine testicular tissue could be injected into the vitreous of visually impaired eyes without causing any serious ocular side effects. Test materials and methods
[0121] Balanced salt solution (BSS) obtained from Allergan Pharmaceutical (Irvine, CA) was used as a placebo. BSS contains 0.64% sodium chloride, 0.075% potassium chloride, 0.048% calcium chloride dihydrate, 0.03% magnesium chloride hexahydrate, 0.39% sodium acetate trihydrate, enough sodium hydroxide to adjust the pH to 7.1-7.2 / hydrochloric acid, and water for injection (appropriate amount 100%). A 30 microliter aliquot of BSS or Hyaluronidase Specific Formulation D (Table 6) was filled in a 300 microsyringe fitted with a 0.5 inch long 29 gauge needle. The sample is then injected into the vitreous of the patient's eye using a microsyringe containing the injected samp...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com