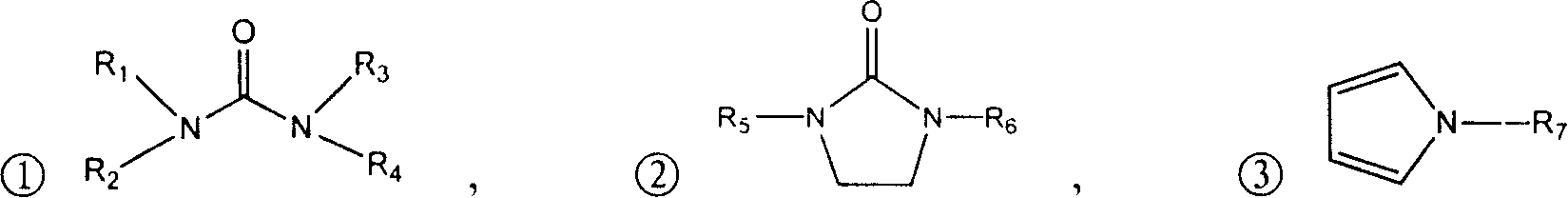
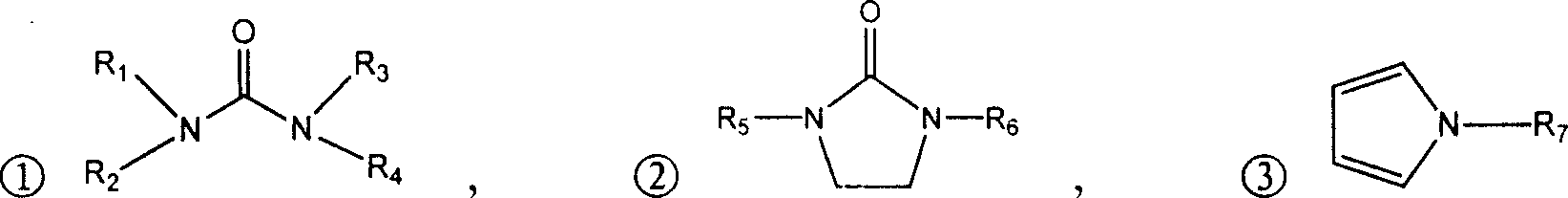
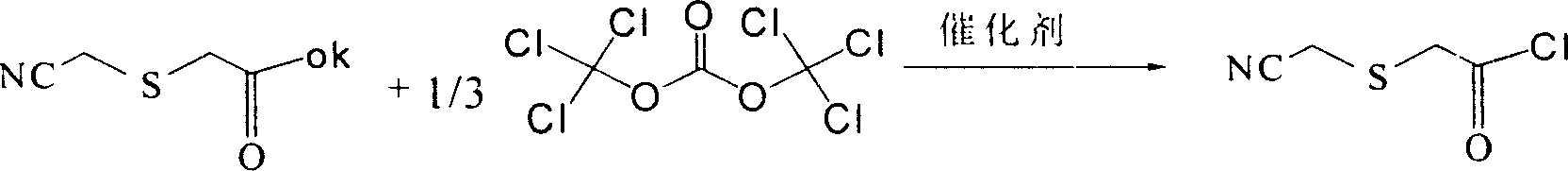
Process for chemical synthesis of 2-(cyanomethyl) thio acetyl chloride
A technology of cyanomethylthioacetyl and cyanomethylthio, which is applied in the field of chemical synthesis of 2-cyanomethylthioacetyl chloride, can solve the problems of potential safety hazards, environmental pollution and high cost, and achieves a reduction in dosage, The effect of reducing production costs and reducing the amount of three wastes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] The feeding molar ratio is: potassium 2-cyanomethylthioacetate: bis(trichloromethyl) carbonate: catalyst tetrabutylurea=1: 0.35: 0.001, wherein potassium 2-cyanomethylthioacetate (C 4 h 4 NKO 2 S) 8.45g, two (trichloromethyl) carbonate (C 3 o 3 Cl 6 )5.2g, tetrabutylurea (C 17 h 36 N 2 (2) 0.02g, organic solvent dichloromethane 126.75g, consumption is 15 times of 2-cyanomethylthioacetic acid potassium.
[0040] In a 150 ml three-necked flask equipped with a thermometer, a reflux condenser, a drying tube, a constant pressure dropping funnel, and a mechanical stirrer, add potassium 2-cyanomethylthioacetate and an organic solvent, start stirring, and cool in an ice-salt bath to - 5°C, then, bis(trichloromethyl)carbonate and catalyst tetrabutylurea were added under ice-salt bath cooling. After the addition is complete, continue to stir for 2 minutes while cooling in the ice-salt bath, then remove the ice-salt bath, slowly raise the temperature to 55-60°C, and stir t...
Embodiment 2
[0042] The molar ratio of feeding is: 2-potassium cyanomethylthioacetate: bis(trichloromethyl) carbonate: catalyst tetrabutylurea=1: 0.5: 0.005, wherein 10.1 g of potassium 2-cyanomethylthioacetate, Bis(trichloromethyl)carbonate 8.9g, tetrabutyl urea 0.08g, organic solvent is 181.8g of dichloromethane, the consumption is 18 times of potassium 2-cyanomethylthioacetate.
[0043] In a 150 ml three-necked flask equipped with a thermometer, a reflux condenser, a drying tube, a constant pressure dropping funnel, and mechanical stirring, add 2-cyanomethylthioacetic acid potassium and dichloromethane, start stirring, and cool in an ice-salt bath to -5°C, then, bis(trichloromethyl)carbonate and tetrabutylurea were added under ice-salt bath cooling. After the addition is complete, continue stirring for 3 minutes while cooling in the ice-salt bath, then remove the ice-salt bath, slowly heat up to 55-60°C, and stir and react at 55-60°C for 12 hours. After the reaction was over, the solve...
Embodiment 3
[0045]The feeding molar ratio is: potassium 2-cyanomethylthioacetate: bis(trichloromethyl) carbonate: catalyst N, N'-dimethylimidazolidinone=1:0.5:0.005, wherein 2-cyanomethyl Potassium thioglycolate 8.45g, bis(trichloromethyl)carbonate 7.4g, N,N'-dimethylimidazolidinone (C 4 h 10 N 2 (0) 0.03g, organic solvent is tetrahydrofuran 143.65g, and consumption is 17 times of 2-cyanomethylthioacetic acid potassium.
[0046] In a 150 ml three-neck flask equipped with a thermometer, reflux condenser, drying tube, constant pressure dropping funnel and mechanical stirring, add potassium 2-cyanomethylthioacetate and tetrahydrofuran, start stirring, and cool to -5 in an ice-salt bath. °C, then, bis(trichloromethyl)carbonate and N,N'-dimethylimidazolidinone were added under ice-salt bath cooling. After the addition is complete, continue to stir for 2 minutes under ice-salt bath cooling, then remove the ice-salt bath, slowly heat up to 55-60°C, and stir and react at 55-60°C for 12 hours. ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com