Synthesis of aluminium riched AFI zeolite
A zeolite, sodium chabazite technology, applied in the direction of crystalline aluminosilicate zeolite, molecular sieves and alkali exchange compounds, other chemical processes, etc., can solve the problems of low silica, no teaching, no teaching high purity, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050] Embodiment 1 synthesizes high-quality Na-type gmelinite
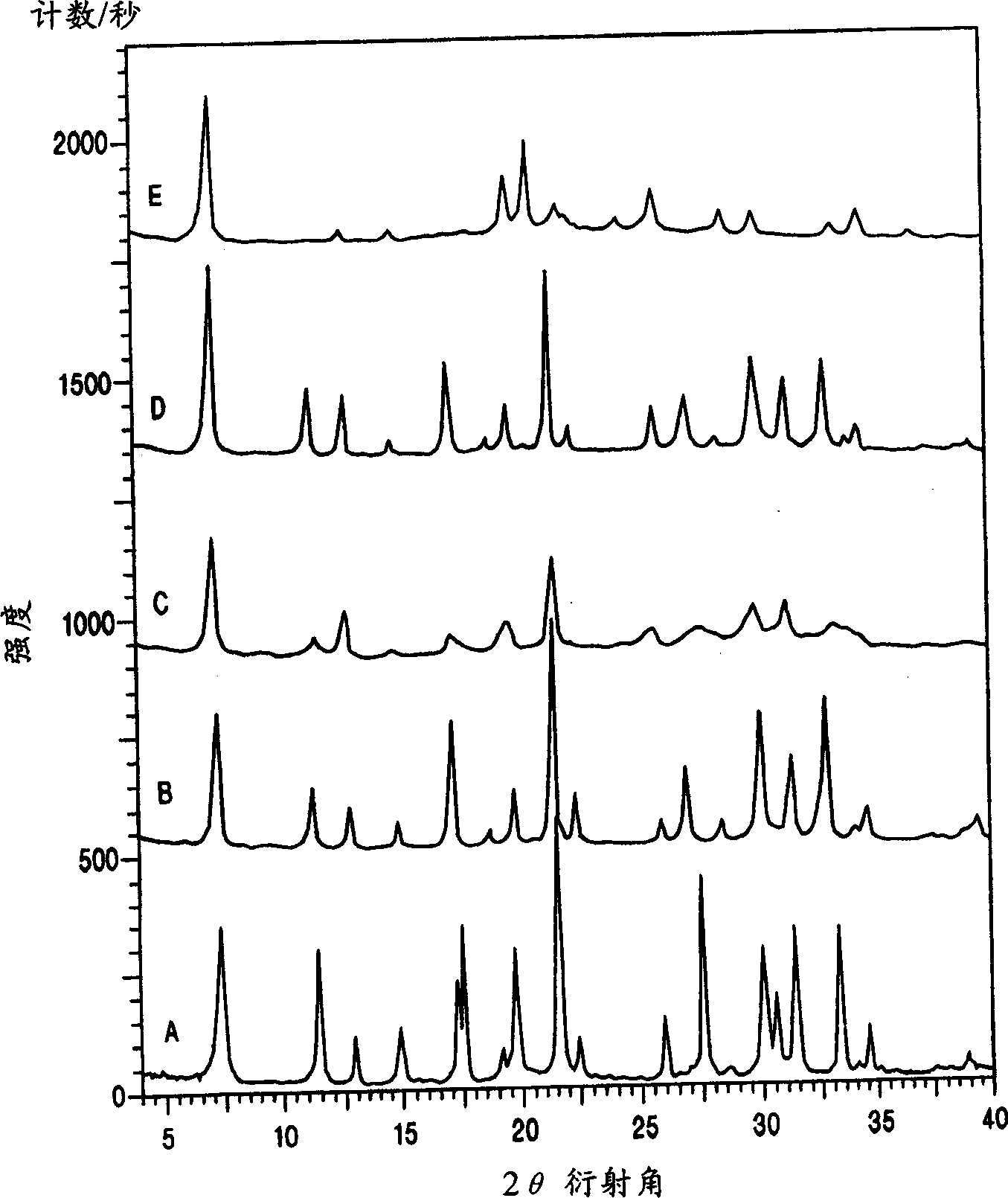
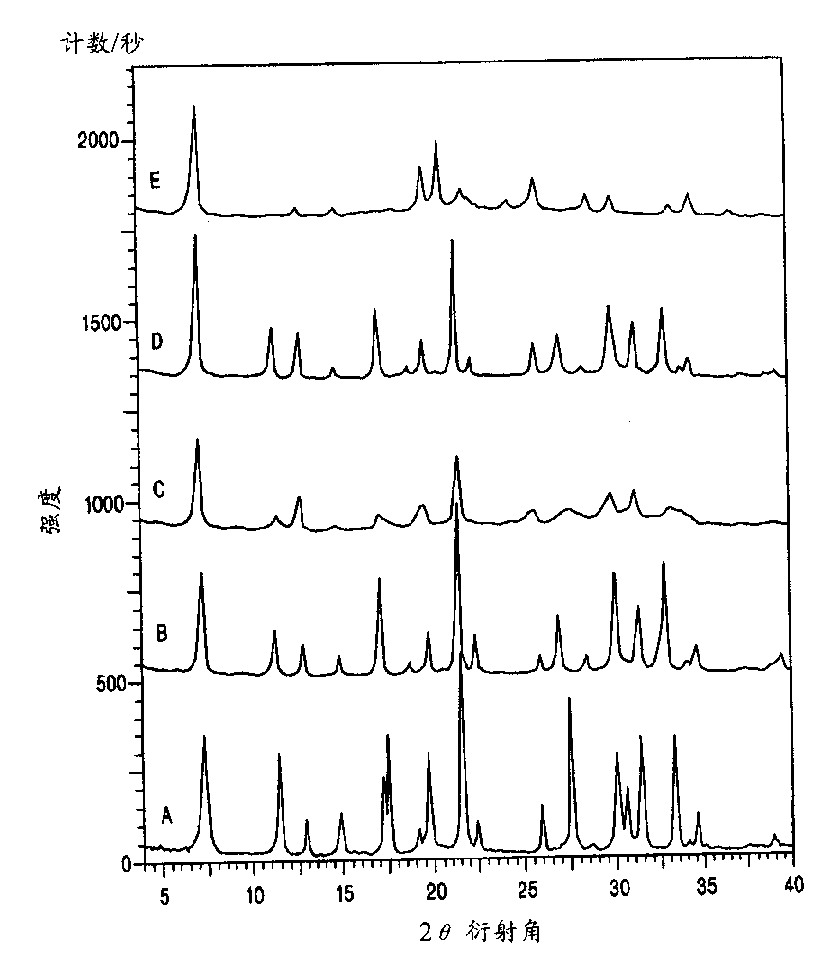
[0051] 5.42 Dab-4Br: 1 Al 2 o 3 : 16.7 Na 2 O: 30 SiO 2 : 570H 2 The gel composition of O was prepared as follows: First, by dissolving aluminum hydroxide hydrate (54% Al 2 o 3 , Aldrich), to prepare sodium aluminate solution (0.4mol / kg Al 2 o 3 and 4mol / kg of NaOH), then 25.0g of sodium aluminate solution was mixed with 54.2g of template Dab-4Br solution (16.4% by weight), and then 66.6g of sodium silicate solution (27% SiO 2 , 14% NaOH, Aldrich). The reaction mixture was stirred for 5 minutes at room temperature, the mixture was transferred to a Teflon bottle and heated in a convection oven at 80°C for 12 days. The product was filtered, washed with water, and then dried in an oven at 100°C. XRD analysis showed high quality Na-type gmelinite ( figure 1 A). Elemental analysis showed that SiO 2 / AlO 3 The ratio of = 4.6. The final product also contains organic species derived from organic templates....
Embodiment 2
[0052] Embodiment 2 prepares Li-GME
[0053] The Na-type gmelinite sample prepared as in Example 1 was contacted with an aqueous solution containing 2 mol / kg KOH and 1 mol / kg KCl at 90° C. for 1 day. It is expected that the molar concentration of the mixture of inorganic cations to be exchanged must be at least 10%. In this particular case, the molar concentration of potassium ions must be at least 10%. Repeat this ion exchange process 3 times in succession. The use of KOH is optional but desirable because KOH dissolves amorphous and other impurities. The product was filtered, washed with water, and then dried in an oven at 100°C. Based on K ion-exchanged gmelinite characteristic peaks, no impurity peaks, and low baseline, XRD analysis shows high-quality K ion-exchanged gmelinite (refer to figure 1 B).
[0054] The K ion-exchanged gmelinite was calcined at 500° C. in air for 5 hours to remove the organic template. XRD analysis confirms that product is gmelinite (with ref...
Embodiment 3
[0056] Embodiment 3 synthetic AFI
[0057] A sample of gmelinite prepared as described in Example 1 was calcined at 500°C in air for 5 hours. XRD analysis shows that product has AFI structure ( figure 1 E). The rate of heating had no significant effect.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com