Tissue augmentation material and methods
A tissue enhancement and tissue technology, applied in the field of filling soft tissue voids or producing soft tissue blisters, urethral sphincter reinforcement materials, can solve problems such as respiratory blockage and unsatisfactory sound quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0098] The preparation of embodiment 1 gel
[0099] A mixture of 15% glycerin, 85% water (by total weight of glycerin and water) and 3.25% NaCMC (again by total weight of liquid ingredients) was prepared as follows:
[0100] 9.303 grams of glycerin and 2.016 grams of NaCMC were mixed in a container. The mixture was then slowly added to 52.718 grams of agitated water in a vessel large enough to hold the batch and allow mixing, using an electric mixer, and stirred at medium speed for 30 minutes. The gel was allowed to stand for at least 4 hours.
Embodiment 2
[0101] Embodiment 2 Preparation of Enhanced Composition
[0102] The glycerol / NaCMC hydrogel (44.04 grams prepared in Example 1) was placed in a mixing vessel large enough to hold a batch. Smooth round and substantially spherical CaHA particles (55.99 g) with a uniform particle size of 75-125 microns were mixed thoroughly with an electric mixer at low speed for 5 minutes until a gel-like homogeneous suspension with uniform distribution of all particles was obtained. The mixed material was filled into 3 ml polysulfone cartridges and sterilized in an autoclave at a temperature of 121°C for 60 minutes.
Embodiment 3
[0103] Preparation of embodiment 3 reinforcing material composition
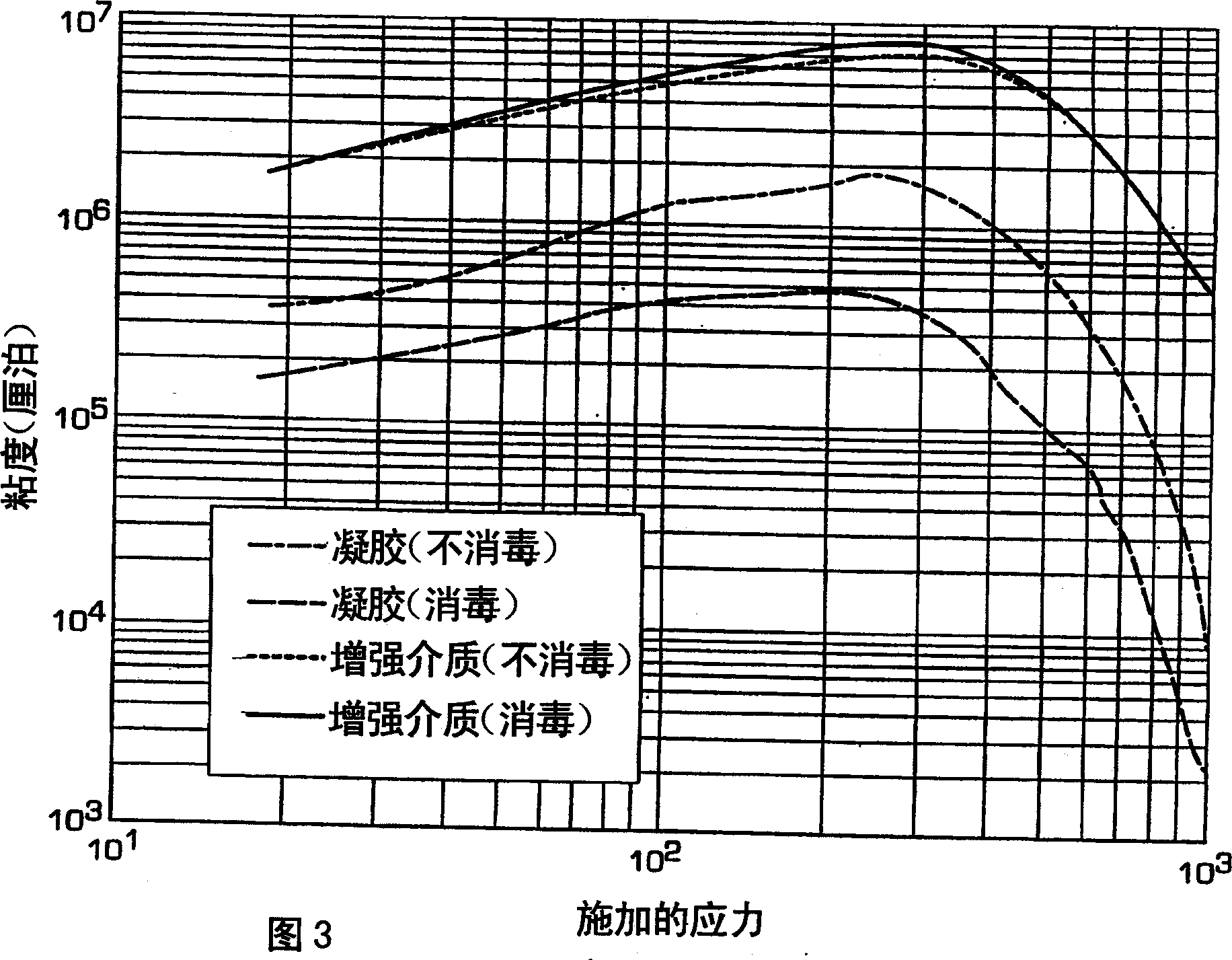
[0104]
prepared gel
increase in preparation
strong composition
increase in disinfection
strong donor
Viscosity (Cp)@500Pa stress
603000
4610000
4340000
Elastic modulus (100Pa@1Hz)
408
2520
2684
Tangent δ(100Pa@1Hz)
0.461
0.453
0.429
gamma 最大
2.227
0.367
0.345
%recover
44.99
45.50
46.96
[0105] A mixture of 25% glycerol, 75% water and 2.25% NaCMC (by total weight of glycerol and water) was prepared as follows:
[0106] 87.90 grams of glycerin and 7.91 grams of NaCMC were mixed in a container large enough to hold the total amount of material. The mixture was then slowly added to 263.71 grams of agitated water in an adequately large container using an electric mixer at medium speed for 30 minutes. The gel was allowed to stand for at least 4 hours.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Viscosity | aaaaa | aaaaa |
| Viscosity | aaaaa | aaaaa |
| Viscosity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com