Process for preparing slow-releasing indapamide tablet
A technology for indapamide and its manufacturing method, which is applied in the field of manufacturing indapamide sustained-release tablets, can solve problems such as high cost, complex production process, and unsuitability for popularization and application, and achieve low cost, low investment, and easy control Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
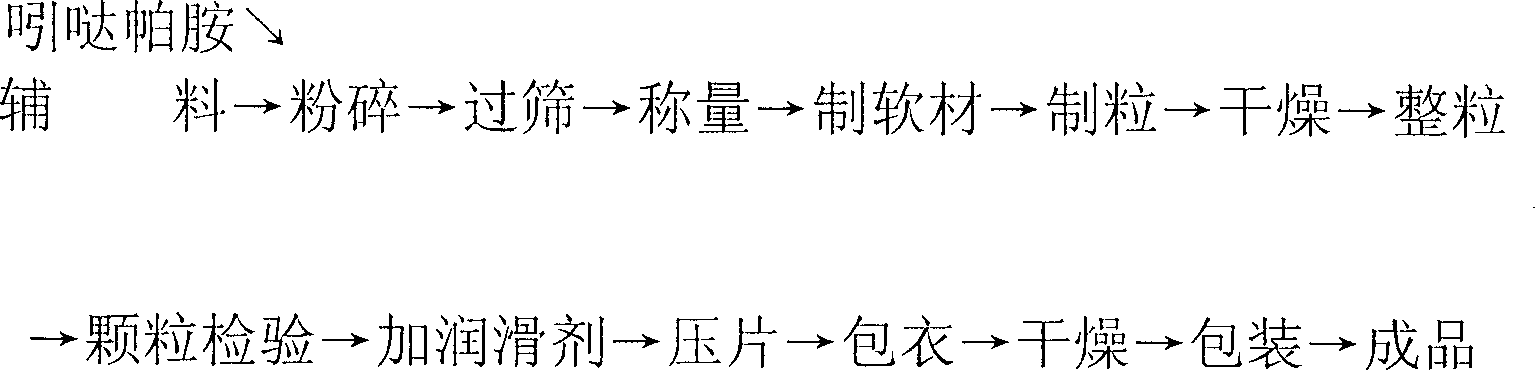
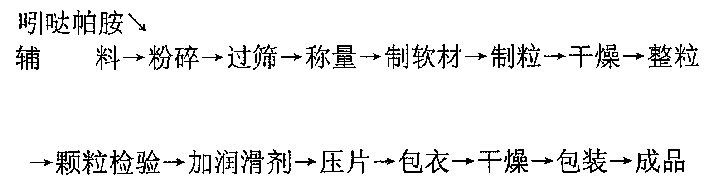
[0010] Based on 1000 indapamide sustained-release tablets, take 1.5 g of indapamide that was pulverized and screened through a 200-mesh sieve, and weigh 100 g of hydroxypropyl methylcellulose, 30 g of Crystalline cellulose, 10g sodium carboxymethyl starch and 60g sucrose, put these raw materials in a mixer and mix evenly, and make soft material with water, then use 30-mesh nylon mesh to make granules with less fine powder and neat without long strips . Dry the granules at a temperature of 60±2°C. After drying, sieve and size the granules to remove oversized particles, then add 10g of magnesium stearate screened through a 100-mesh sieve and mix evenly. Tablets were made into 1000 core tablets containing 1.5 mg of indapamide. At the same time, weigh 3g of methyl cellulose, add 70ml of distilled water under stirring conditions, and use ultrasonic treatment, then add 70ml of distilled water until the solution is clear, then add 15g of talcum powder and 8g of titanium dioxide and ...
Embodiment 2
[0012] Based on 1000 indapamide sustained-release tablets, 1.5g indapamide, 10g methylcellulose, 30g hydroxypropylcellulose, 5g lactose, 15g mannitol, 40g modified starch and 1g magnesium stearate Add-on and the same process of embodiment 1 are made into tablet cores. Simultaneously according to 20g hydroxyethyl cellulose, 8g talcum powder, 1g titanium dioxide, 5ml macrogol 400, 140ml distilled water and the ethanol add-on that adds to 500ml coating solution at last and make coating solution with the technique identical with embodiment 1 . The method of coating treatment is also the same as in Example 1.
Embodiment 3
[0014] Based on 1000 indapamide sustained-release tablets, according to the addition amount and embodiment of 1.5g indapamide, 60g methylcellulose, 30g sodium alginate, 70g lactose, 30g modified starch and 8g magnesium stearate 1 The core is made by the same process. Simultaneously according to 8g hydroxypropyl cellulose, 2g talcum powder, 15g titanium dioxide, 5ml macrogol 400, 140ml distilled water and the ethanol add-on that is finally added to 500ml coating solution and the process identical with embodiment 1 makes coating solution . The method of coating treatment is also the same as in Example 1.
[0015] A low-dose antihypertensive sustained-release drug containing 1.5 mg of indapamide active ingredient can be produced by using the manufacturing method of the indapamide sustained-release tablet provided by the present invention, and the patient only needs to take one tablet per day to reach the therapeutic concentration. Compared with low-dose indapamide sustained-rel...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com