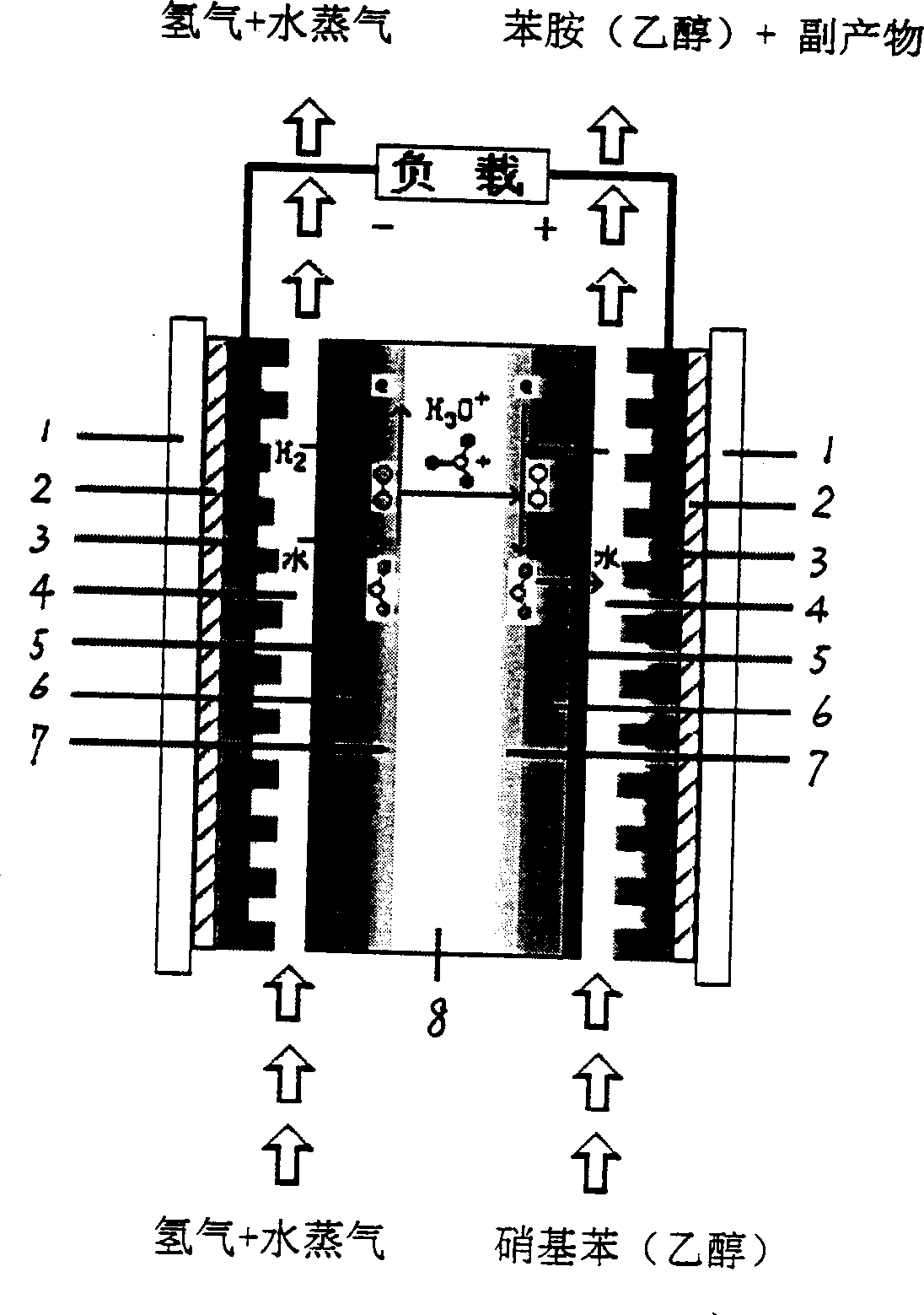
Aniline-synthesizing and electric energy-generating process utilizing proton exchange film fuel cell
A proton exchange membrane and fuel cell technology, applied in the electrolysis process, power generation process, etc., can solve the problems of harsh reaction conditions and serious energy consumption, and achieve the effects of high utilization value, simple production process and low pollution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0019] Example 1: Preparation of platinum loading 0.5mg / cm 2 The membrane electrode, and the membrane electrode into the battery. Prepare 30ml of 0.25mol / L nitrophenylethanol solution, add 1g of electrolyte, connect the cathode and anode pipelines, and control the battery temperature at 30°C. After the initial polarization, hydrogen gas was introduced, and the flow rate of hydrogen gas was controlled at 20ml / min. The peristaltic pump was turned on, and the flow rate of catholyte was controlled at 1ml / min. The measured open circuit voltage is 0.25V, 0.58mmol of aniline can be obtained in 2 hours, the conversion rate is 19%, and the maximum power density is 3.1mW / cm 2 , the current density at this time is 22mA / cm 2 .
example 2
[0020] Example 2: Preparation of platinum loading 2mg / cm 2 The membrane electrode, and the membrane electrode into the battery. Prepare 30ml of 2mol / L nitrophenylethanol solution without adding electrolyte, connect the cathode and anode pipelines, and control the battery temperature at 70°C. After the initial polarization, hydrogen gas was introduced, and the flow rate of hydrogen gas was controlled at 80ml / min. The peristaltic pump was turned on, and the flow rate of catholyte was controlled at 6ml / min. The measured open circuit voltage is 0.28V, 0.64mmol of aniline can be obtained in 2 hours, the conversion rate is 21%, and the maximum power density is 3.6mW / cm 2 , the current density at this time is 27mA / cm 2 .
example 3
[0021] Example 3: Adopt a typical proton exchange membrane fuel cell porous gas diffusion three-in-one membrane electrode, and install the membrane electrode into the battery. Prepare 30ml of 0.5mol / L nitrophenylethanol solution, add 1g of electrolyte, connect the cathode and anode pipelines, and control the battery temperature at 50°C. After the initial polarization, feed hydrogen, control the flow rate of hydrogen at 10ml / min, turn on the peristaltic pump, control the flow rate of catholyte to 3ml / min, measure the open circuit voltage 0.31V, obtain aniline 0.78mmol in 2 hours, the conversion rate is 29%, the maximum The power density is 4mW / cm 2 , the current density at this time is 30mA / cm 2 .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com