17 peptide organic compound and its application
An organic compound and reaction technology, applied in the field of polypeptide organic compounds and organic compounds, can solve problems such as blood transfusion reactions, achieve the effect of avoiding side effects and inhibiting hyperacute rejection
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Example 1: Using the anti-B blood monoclonal antibody as the target protein, a positive phage clone was obtained by the method of phage display.
[0021] The phage display method uses genetic engineering to clone a set of synthetic oligonucleotide fragments of a certain length and random sequence into a specific expression vector, so that the expression product is presented on the surface of filamentous phage in the form of fusion protein. Since the peptide library contains all possible amino acid sequences of small peptides of this length or most of them, each phage presents one of the peptides, so the library has a large capacity and is easy to screen and amplify. Infect E. coli with the phage peptide library, and the random oligonucleotide fragments recombined into the phage are replicated in E. coli and expressed in the coat protein of the phage. Then, coat the target protein on the microtiter plate. After mixing the phage peptide library with the target protein, w...
Embodiment 2
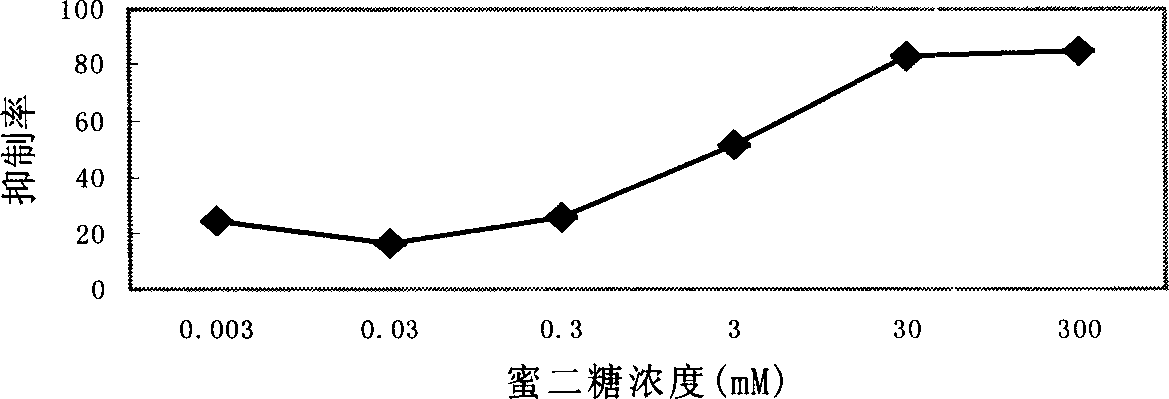
[0024] Embodiment 2: Competitive ELISA (melibiose) test
[0025] Test method: Coat 96-well ELISA plate with 100 μL / well of anti-B blood monoclonal antibody (titer 1:128), overnight at 4°C. 5% BSA was blocked at room temperature for 2 hours, and the plate was washed 3 times with 5 mM TBS buffer solution. The ELISA solution added was 100 μL of a mixture of different concentrations of melibiose and phage stock solution. The following steps are the same as before. Calculated inhibition rate=(OD450 without melibiose-OD450 with melibiose) / OD450*100% without melibiose
[0026] Test results: Melibiose can very well inhibit the combination of anti-B blood monoclonal antibodies and positive clones, and the effect can be seen at a concentration as low as 0.003mM. With the increase of the concentration of melibiose, the concentration of 300mM The inhibition rate has reached more than 80%, which shows that the positive clones are likely to bind to the site where melibiose binds to the an...
Embodiment 3
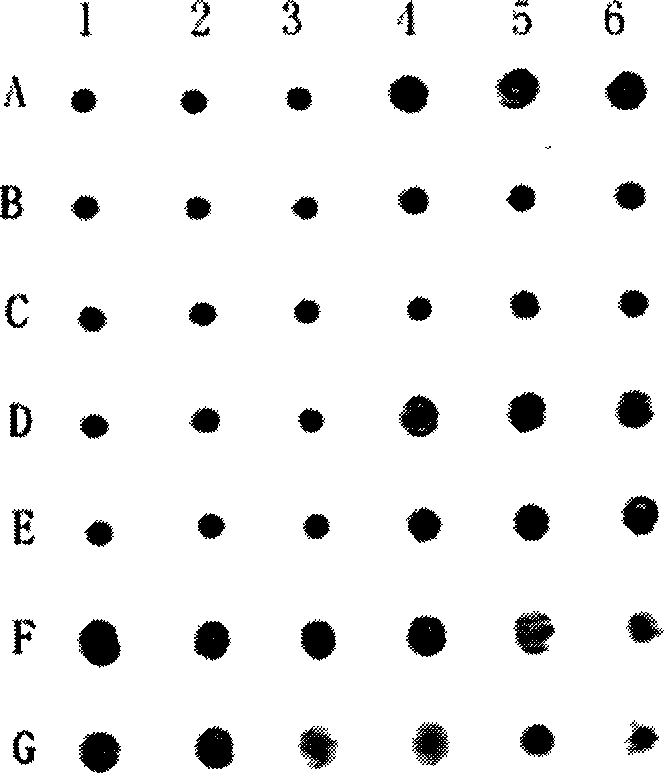
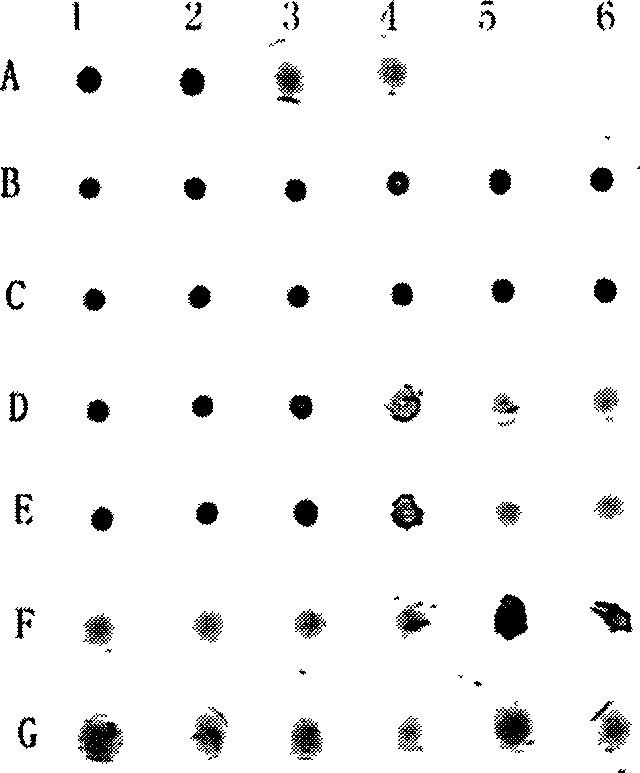
[0028] Example 3: Small peptide inhibits the agglutination reaction between type A human serum and pig erythrocytes
[0029] Test method: Suspend the prepared fresh 2% porcine red blood cells in the phosphate buffer solution with pH=7.4 for later use. In the wells of the V-type hemagglutination plate, after 40ul of small peptides are sequentially diluted well by well with phosphate buffer, 40ul of appropriately diluted blood type A human serum and 40ul of 2% porcine red blood cells are added to each well. Then shake the 120ul mixture with a shaker for 1 minute at room temperature, and then let it stand for 1 hour to observe the agglutination result. If the red blood cells settle to the bottom of the well as a round spot with smooth edges, this is not agglutinated. If the red blood cells do not sink to the bottom of the well, but appear as a network with rough edges, this is called agglutination. A blank and a positive control must be set up in the test.
[0030] Test result...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com