Preparations for preventing bile acid diarrhea
A technology for bile acid and diarrhea, which is applied in the field of preparations for preventing bile acid diarrhea
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0060] The first fluid (pH about 1.2) of the Japanese Pharmacopoeia was added to a 10 mL test cylinder containing 2 mL of coated particles to the full scale, and then the swelling rate and volume of the CAS resin were measured over time. The state of the coating was observed by SEM (scanning electron microscope), and the relationship between the swelling rate and the cracking rate of the coating was detected. (Example 1) Preparation of enteric-coated cholestyramine (CSA) preparation (pH-dependent)
[0061] As the enteric layer, HPMCAS-HF (Shin-Etsu Chemical Co., Ltd.) having a dissolution pH of 6.5 or more was used. By using HPMCAS-HF / triethyl citrate / (EtOH / CH 2 Cl 2 , 7:3v / v)=7 / 1 / 92% (weight) of the coating solution can prepare enteric coating particles. Given HPMCAS'-COC 2 h 4 The cause of the interaction between COOH" and trimethylamine of CSA, a barrier layer was inserted between the enteric layer and CSA. The solution was coated with calcium hydrogen phosphate / HPC-...
Embodiment 2
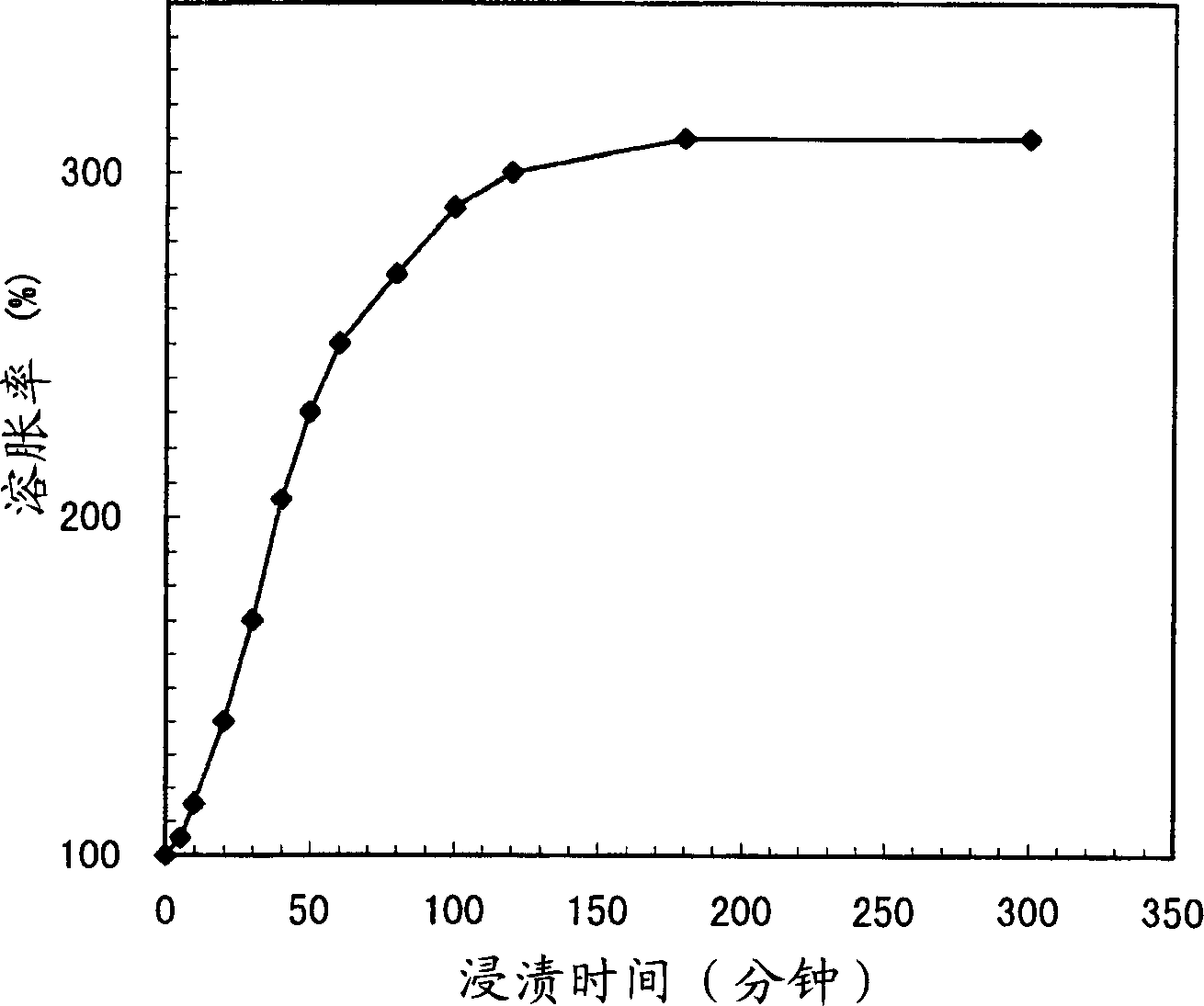
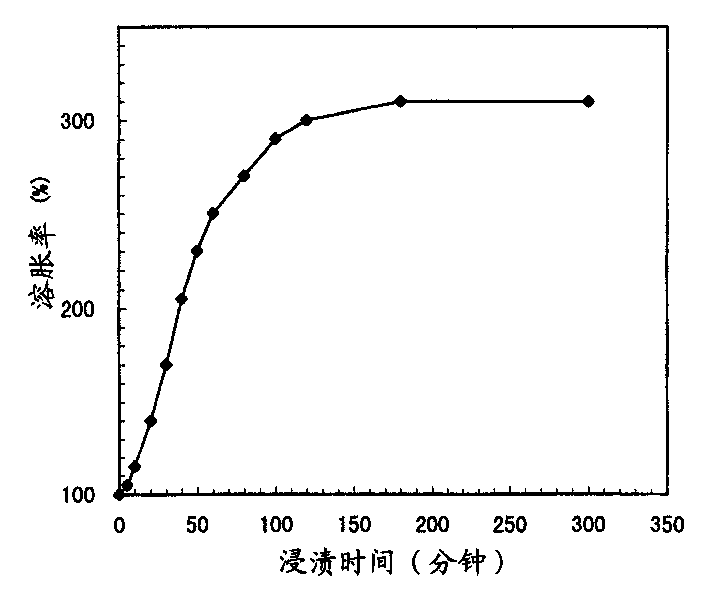
[0069] It is proved that the isolation layer and the enteric coating layer are uniform coating layers respectively, and their contents are respectively 13.6% (coating thickness=about 10 μm) and 30.5% (coating thickness = about 10 μm) and 30.5% (coating thickness = about 30 μm). The results of the immersion test were figure 1 shown. The swelling rate was 170% at 30 minutes, 250% at 1 hour and 300% at 2 hours. The progress of the burst release of the coating layer was confirmed by SEM examination to be about 20% at 30 minutes, about 80% at 1 hour, and 100% at 2 hours. (Example 2) Preparation of Ethylcellulose Coated Cholestyramine (CSA) Formulations (Type Controlled by Coating Thickness)
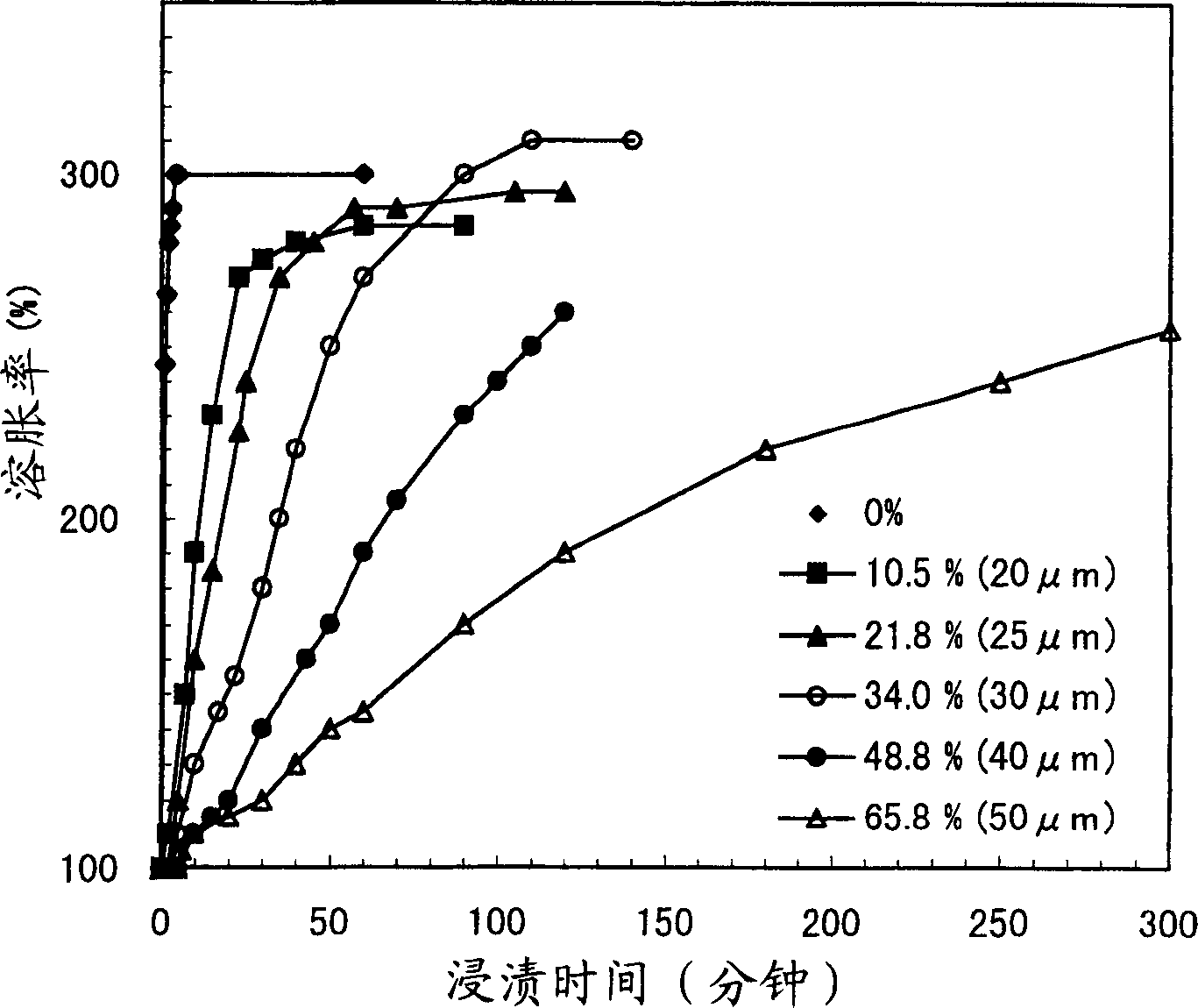
[0070] Five kinds of ethylcellulose (EC) granules were prepared using a coating solution of EC / EtOH=5 / 95% by weight, wherein the coating amount thereof was changed according to the coating time. Its composition is shown below. (Table 2)
[0071] Composition A B C D E
[0072] DOWEX...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com