Human angiogenin antisense oligonucleotide and medicine combination
A technology of antisense oligonucleotides and nucleotides, applied in the field of antisense oligonucleotides of angiogenin, can solve the problems of high non-specific toxicity of cells and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0061] Material acquisition
[0062]The sources of the materials used in the present invention are as follows. Human angiogenin is prepared by recombinant ribonucleic acid method. Basic Fibroblast Growth (bFGF) and Epidermal Growth Factor (EGF) were purchased by Promega, USA; Vascular Endothelial Growth Factor (VEGF) was purchased by R and D, USA. The special medium for vascular endothelial cells (HE-SFM) and the cell transformation reagent Lipofectin were purchased from Gibco / BRL, USA. Ribonuclease (RNase A), Deoxyribonuclease (DNase I) and Proteinase K (Protease K) were purchased from Sigma, USA. Human placental venous vascular endothelial cells (HUVE) and smooth muscle cells (SMC) were purchased by American Cell System; human colon adenocarcinoma cell HT-29 and human prostate cancer cell PC-3 were purchased by American ATCC; cancer cell culture medium DMEM and fetal bovine serum (FBS) were purchased from Biowhittaker, USA. Nude mice with immunodeficiency for the experiment were...
Embodiment 2
[0064] Cell culture
[0065] Human placental vein endothelial cells (HUVE) are cultured in a special medium for vascular endothelial cells containing 10% fetal bovine serum and 10 ng basic fibroblast growth factor per ml (HE-SFM+10%FCS+10ng / ml bFGF )in. The culture condition is 37 degrees Celsius, constant temperature and humidity, 5% carbon dioxide gas phase. Change the medium every two days and subculture at a ratio of 1:3. Experiment with HUVE cells from the third to fifteenth passages.
[0066] Human placental vein vascular smooth muscle cells, human prostate tumor cells PC-3, and human colon adenocarcinoma cells HT-29 were cultured in DMEM containing 10% fetal bovine serum. Subculture at a ratio of 1:4.
Embodiment 3
[0068] Antisense Oligonucleotide Treatment
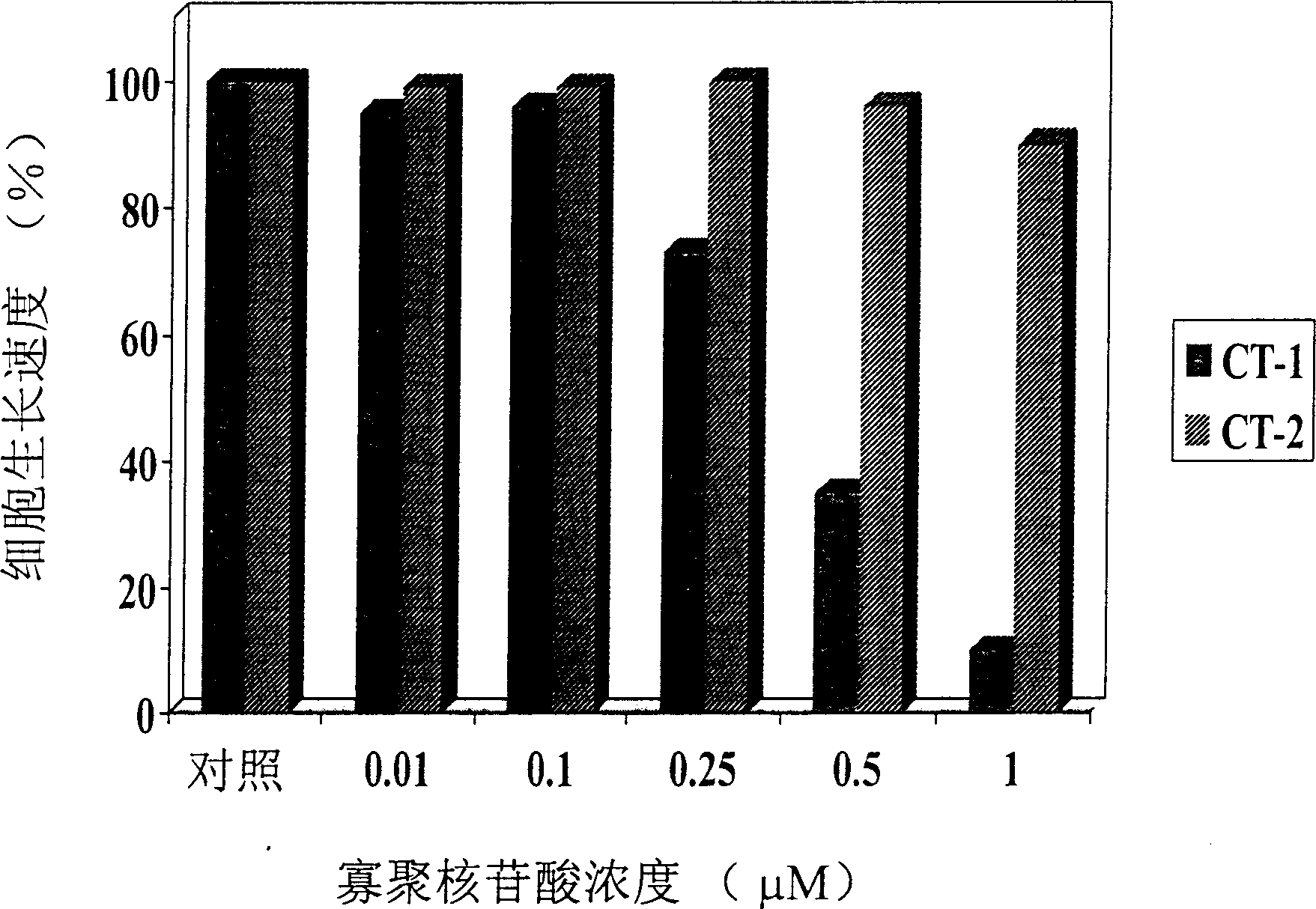
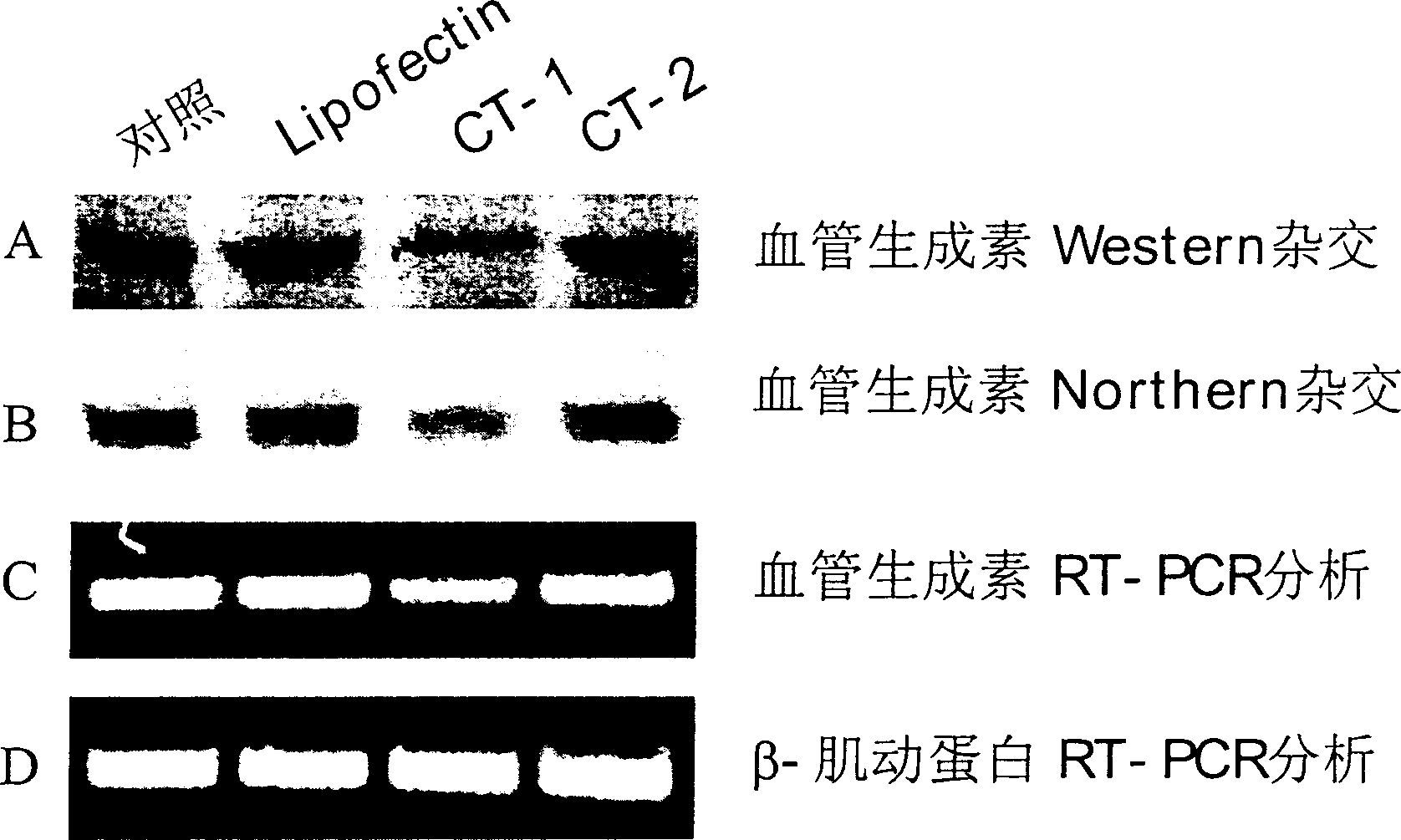
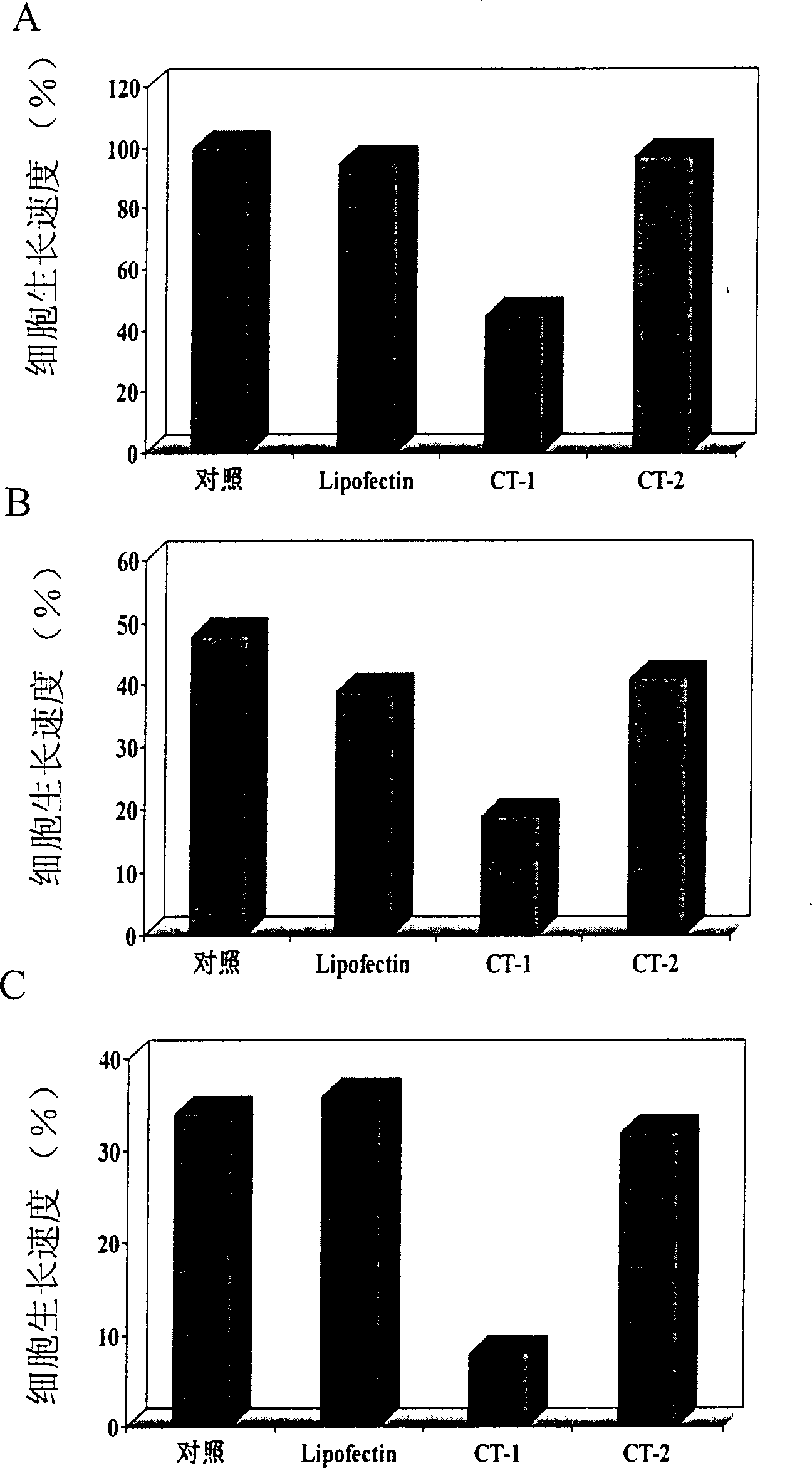
[0069] HUVE cells were treated with trypsin-EDTA, the number of cells was measured with a hemocytometer or Coulter counter, and then cultured in a 35-mm cell culture dish at a density of 100,000 cells per square centimeter. After incubating at 37 degrees Celsius for 24 hours, wash three times with Opti-MEM, so that the cells can be treated with antisense oligonucleotides. Antisense oligonucleotides of different concentrations are mixed with an appropriate amount of lipofectin, a lipofectin, and after pre-incubation at room temperature for 45 minutes, they are added to the above-mentioned cells and incubated at 37 degrees Celsius for 5 hours to 72 hours. In some experiments, cells will be retreated with the same antisense oligonucleotides again every 24 hours. In other experiments, the cells treated with the above-mentioned antisense oligonucleotides were treated with different angiogenic factors for 48 hours to determine the rate of cel...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com