Process for preparing beta-phenylethanol
A technology for phenethyl alcohol and chloroethyl alcohol, applied in the field of preparing β-phenethyl alcohol, can solve the problems of serious equipment corrosion, difficult to recover by-products, large consumption of benzene, etc. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
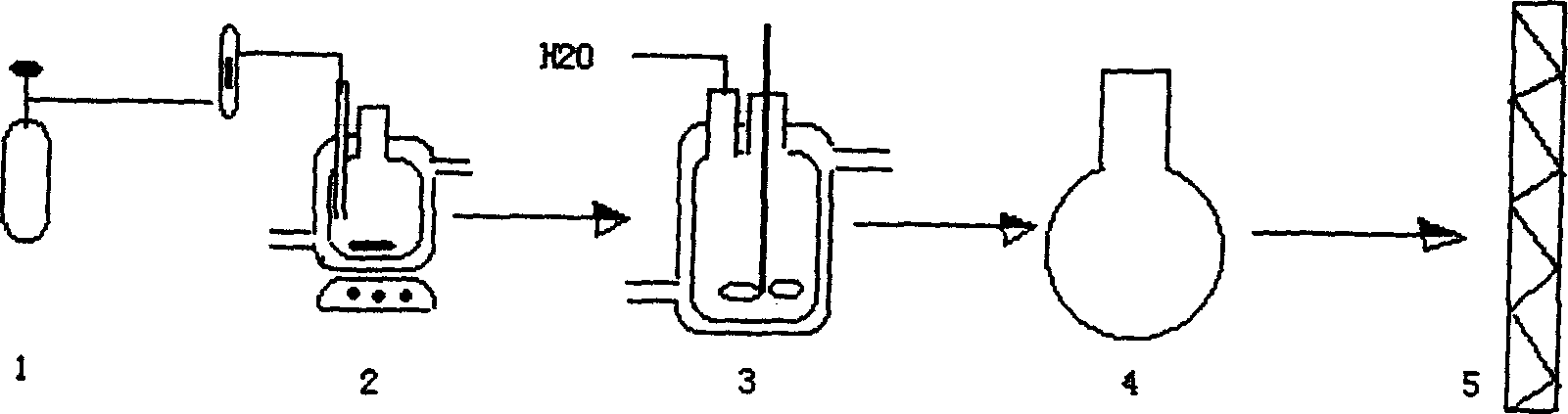
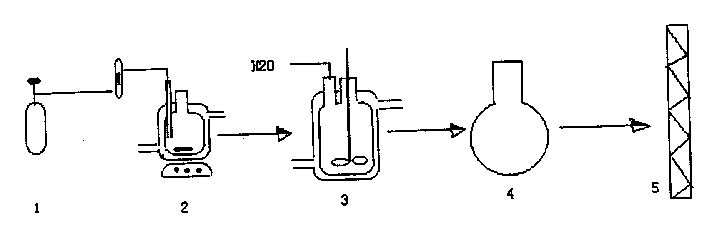
[0026] see figure 1 , the reaction equipment is mainly composed of ethylene oxide storage tank 1, jacketed alkylation reactor 2, jacketed hydrolyzer 3, concentration evaporator 4 and rectifier 5, and the reaction process is: alkylation Reaction → hydrolysis reaction → evaporation and concentration → rectification and purification, and further gel treatment.
[0027] (1) Alkylation
[0028] The alkylation reaction is carried out in an alkylation reactor. The alkylation reactor is a three-necked jacketed glass bottle with a stirring port, a ventilation port and a feeding port, with a volume of 250 milliliters, and 95% ethanol refrigeration circulation fluid is passed through the jacket as a cooling medium. The specific operation is: weigh 129 grams of benzene, put it into a constant weight alkylation reaction bottle, put a magnetic stirrer at the same time, start the circulating refrigerator and electromagnetic stirrer, and reduce the temperature of the reaction system to 5°C-...
Embodiment 2
[0037] (1) Alkylation
[0038] With embodiment 1. Calculate and pass into oxirane total amount 11.3 grams.
[0039] (2) Hydrolysis
[0040] Change the electromagnetic stirring of the above reactor into mechanical stirring, and install a feeding dropping funnel on the vent. Start the agitator, control the stirring speed to 500 rpm, and the hydrolysis temperature to 10-12°C, add 23 grams of deionized water to the reactor dropwise within 30 minutes, until the reaction system generates a transparent organic solution and a white wet gel solid. 140.2 grams of organic liquid in the upper layer was poured out, leaving 52.5 grams of gel in the reaction bottle.
[0041] (3) Evaporation and concentration
[0042] The organic liquid obtained by hydrolysis was transferred to a 250 mL distillation flask. After distillation, 114.6 grams of 99.9% benzene and 24.5 grams of β-phenylethanol concentrate were obtained.
[0043] (4) Distillation and refining
[0044]Pour 24.5 grams of β-phe...
Embodiment 3
[0048] Alkylation
[0049] With embodiment 1. Calculate and pass into oxirane total amount 11.6 grams.
[0050] (2) Hydrolysis
[0051] Change the electromagnetic stirring of the above reactor into mechanical stirring, and install a feeding dropping funnel on the vent. Start the agitator, control the stirring speed to be 500 rev / min, and the hydrolysis temperature is 5-6°C. In 30 minutes, add deionized water into the reactor four times, add 6 grams each time, add 24 grams of water in total, until The reaction system produces a transparent organic solution and a white wet gel solid. 140.3 grams of organic liquid in the upper layer was poured out, and 53.2 grams of gel remained in the reaction bottle.
[0052] (3) Evaporation and concentration
[0053] The organic liquid obtained after hydrolysis was transferred to a 250 mL distillation flask. After distillation, 113.8 grams of 99.9% benzene and 24.6 grams of β-phenylethanol concentrate were obtained.
[0054] (4) Distill...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com