Method for establishing fingerprint of red-rooted salvia root fat-soluble component and its standard map
A technology of fingerprints and establishment methods, which is applied in the establishment of fingerprints of the fat-soluble components of Salvia Miltiorrhiza and its standard spectra, achieving good reproducibility, high precision, and the effect of preventing myocardial infarction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 11
[0026] Embodiment 11. Apparatus and reagent 1.1 Apparatus
[0027] Agilent 1100 high performance liquid chromatograph; Agilent VWD ultraviolet variable wavelength detector; Aotai chromatography star workstation. 1.2 Reagent
[0028] Tanshinone IIA (for content determination) was purchased from China National Institute for the Control of Pharmaceutical and Biological Products; methanol, chromatographically pure; self-made distilled water; syringe-type microporous membrane filter, organic system, diameter 13mm, 0.45μm; Salvia miltiorrhiza produced in the medicinal material base, commercially available salvia miltiorrhiza, purchased from Tianjin medicinal material company. 2. Methods and results 2.1 Chromatographic conditions
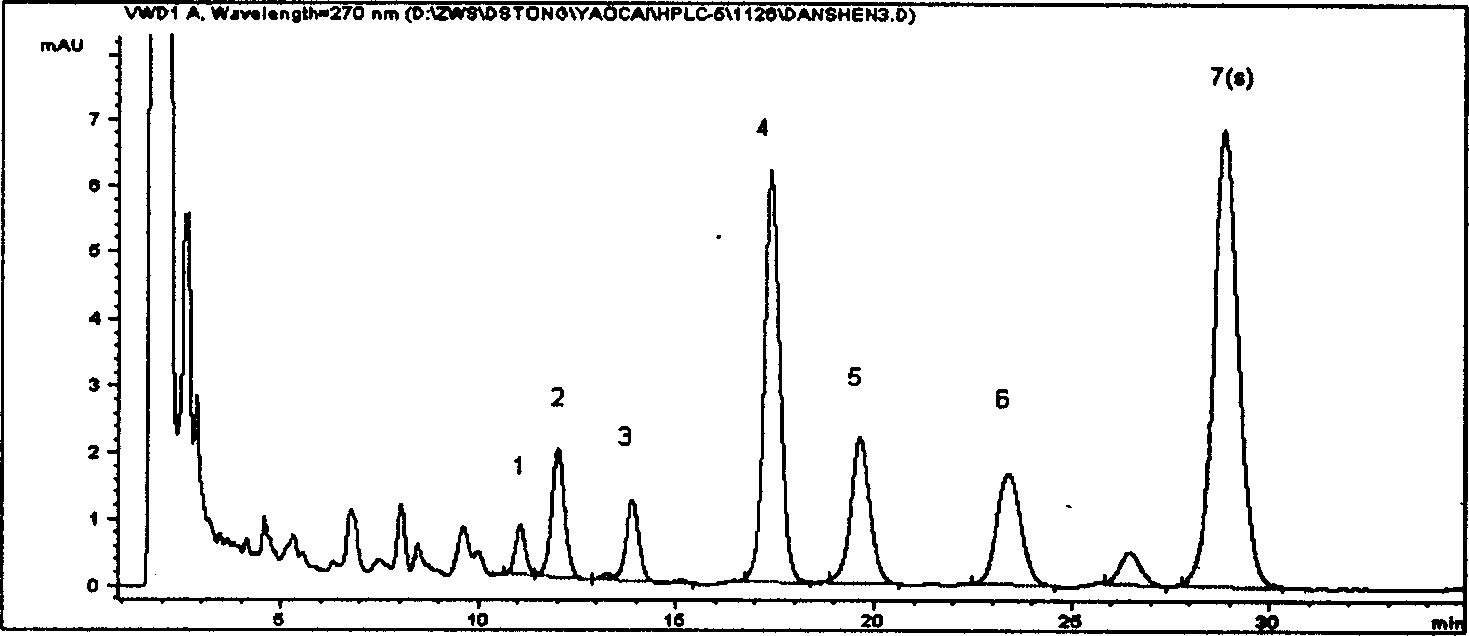
[0029] Chromatographic column: Octadecyl bonded silica gel (4.6×250mm, 5μ) American Aotai Company; mobile phase: methanol: water 3:1; detection wavelength: 270nm; flow rate: 1ml / min; column temperature: 40°C; The number of theoretical plates is calculat...
Embodiment 2
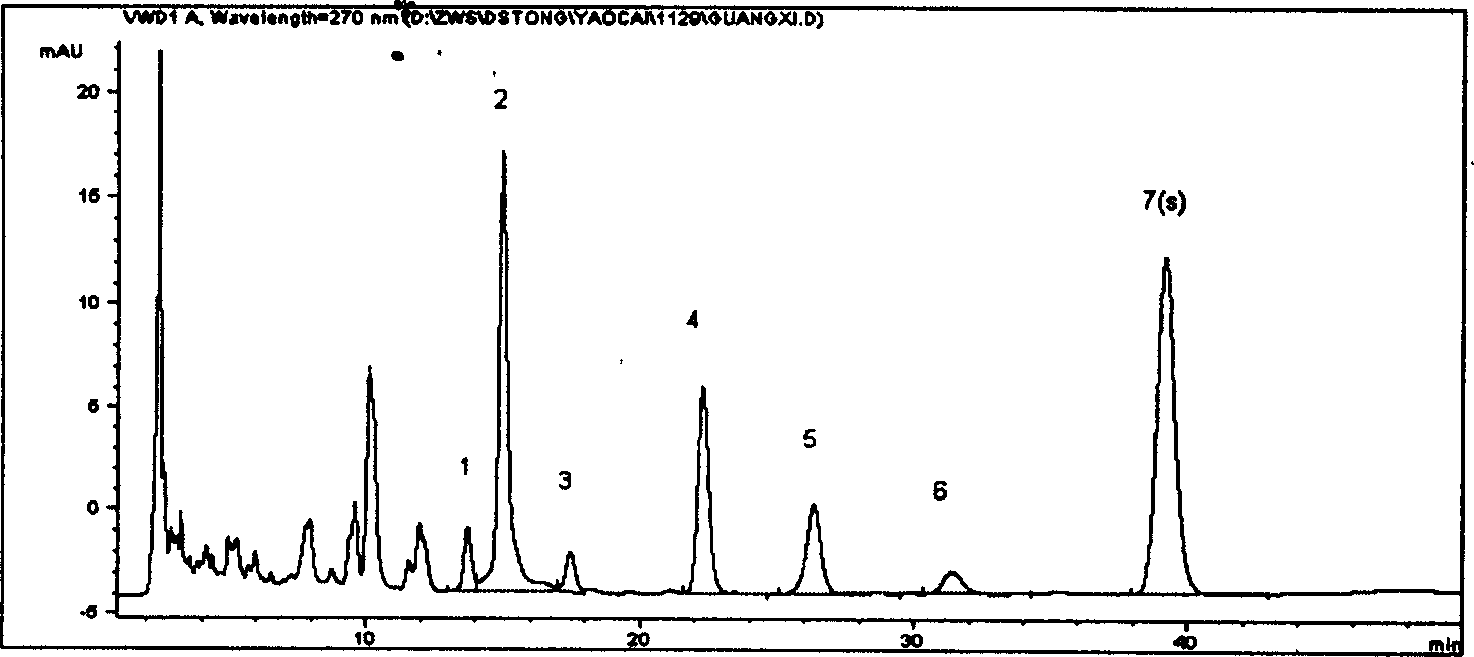
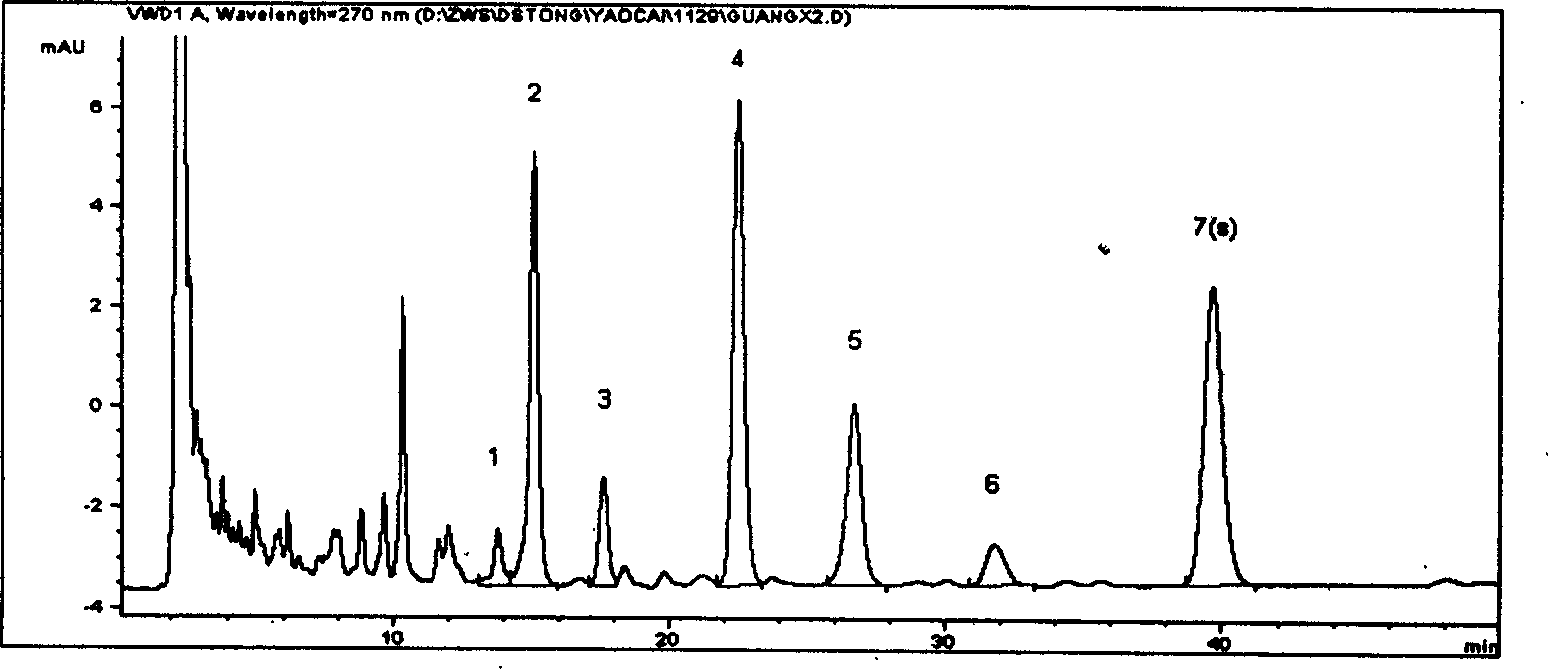
[0048] Preparation of the test solution Take 0.3g of Danshen powder, add 50ml of methanol, sonicate for 25min, let cool to room temperature, filter, and obtain. As the test drug, 10 batches of Salvia Miltiorrhiza produced in Tasly Shangluo Medicinal Material Base were used. Other conditions are with embodiment 1.
[0049] Results: The statistical results of the relative retention time and relative peak area of the common peaks of ten batches of medicinal materials are shown in Table 3 and Table 4 below. Table 3: Statistical batch number\total of relative retention time of 10 batches of Salvia miltiorrhiza
[0050] 1 2 3 4 5 6 7有指纹峰材1104 0.347 0.389 0.445 0.568 0.678 0.815 1材1105 0.351 0.380 0.442 0.566 0.683 0.813 1材1106 0.355 0.387 0.441 0.561 0.682 0.809 1材1107 0.346 0.375 0.443 0.563 0.680 0.806 1材1108 0.346 0.374 0.444 0.565 0....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com