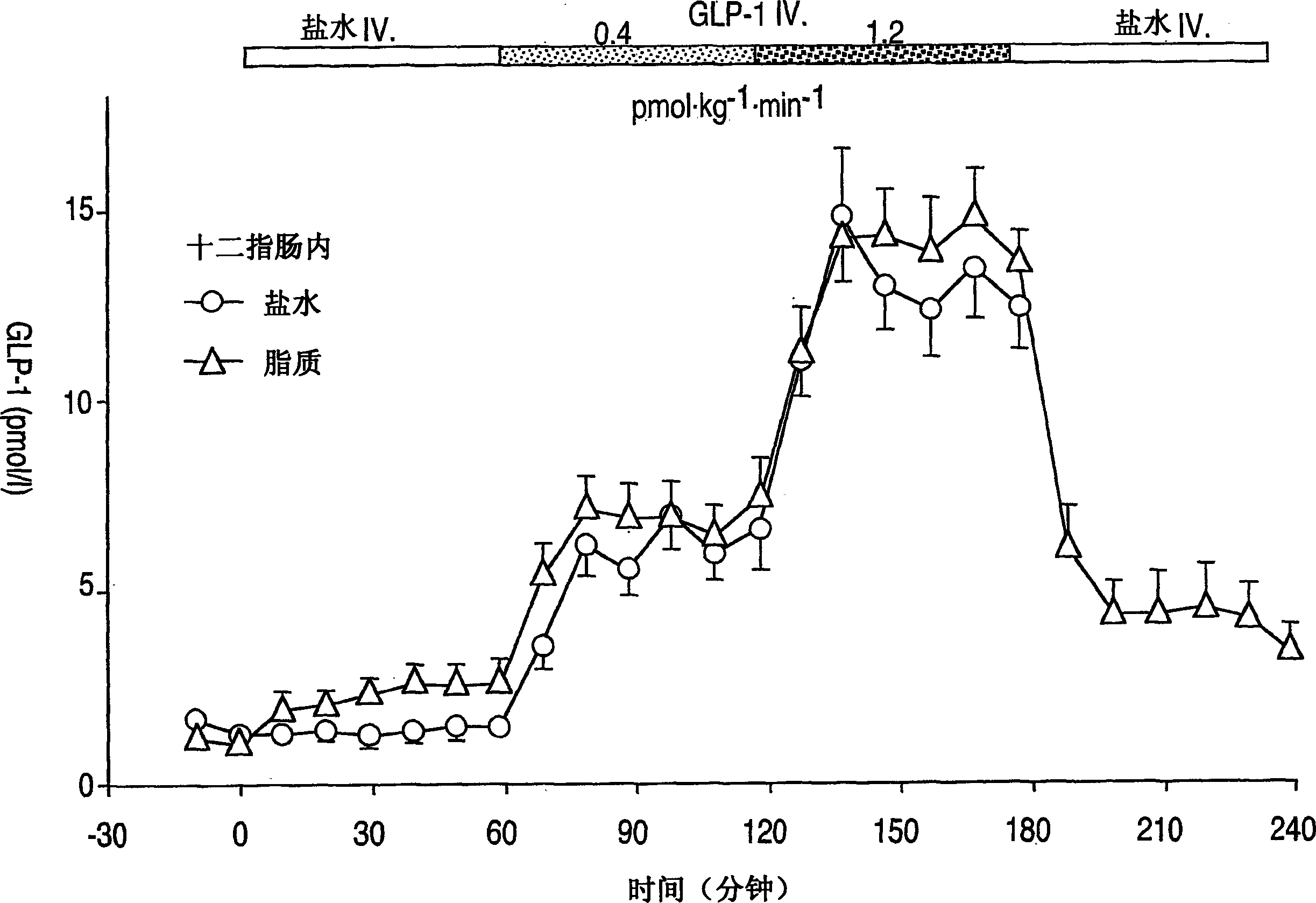
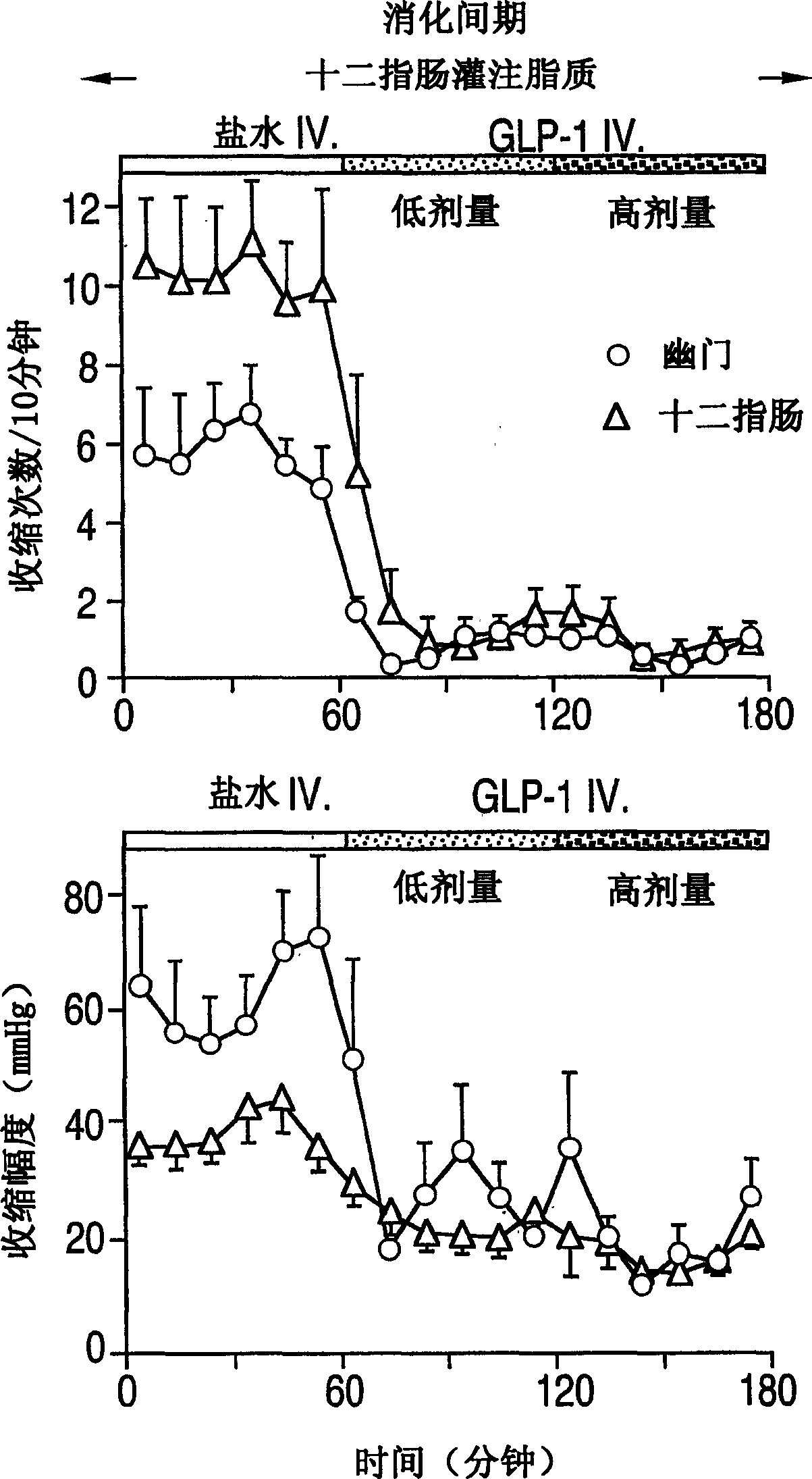
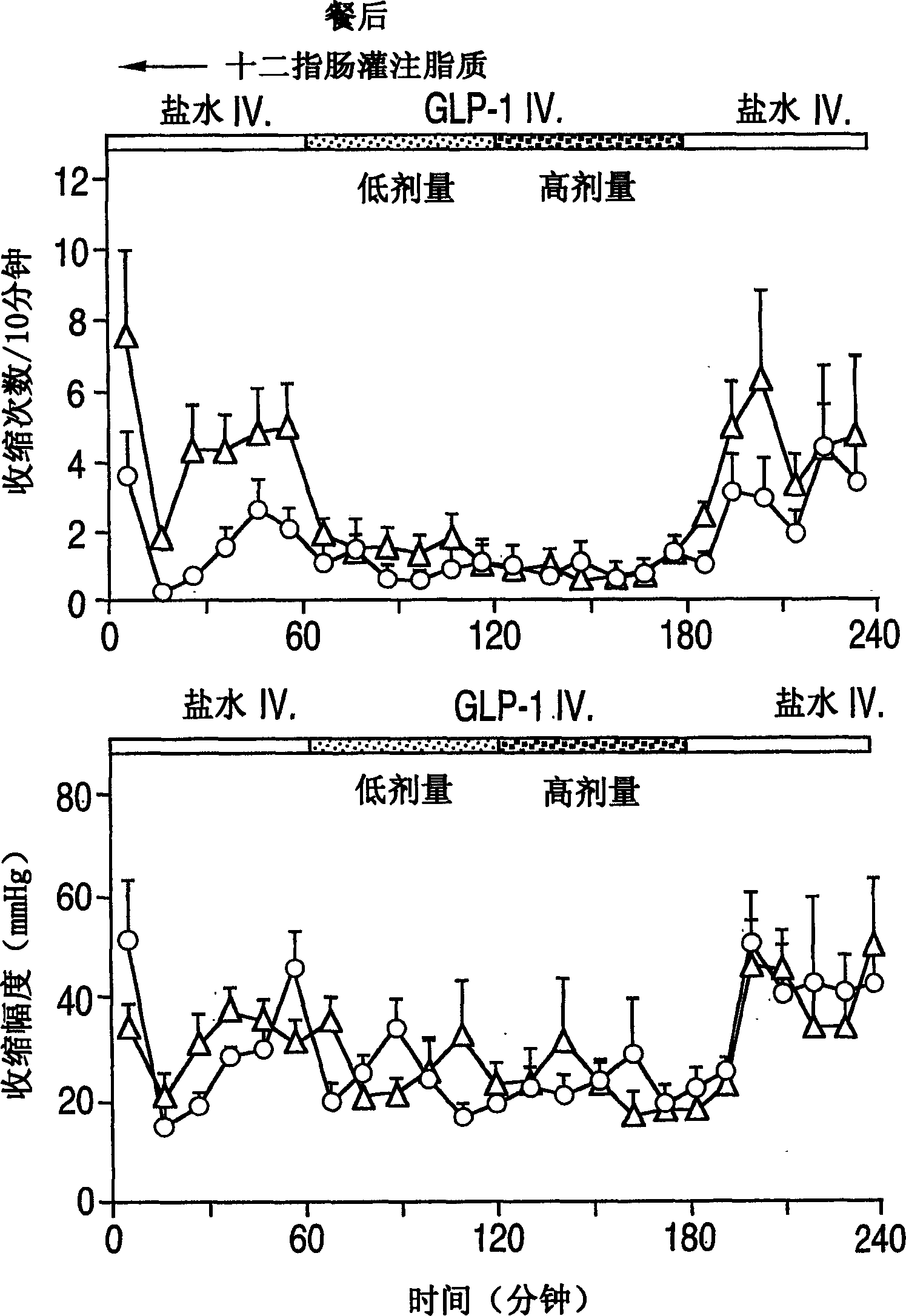
Effects of glucagon-like peptide-1 (7-36) on antro-pyloro-duodenal motility
A technique of duodenum and gastric antrum pylorus is applied in the field of the influence of glucagon-like peptide 1 (7-36) on gastric antrum pylorus and duodenum motility, and can solve adverse side effects, long-term use, internal Problems such as interruption or abort of speculum operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0067] Preparation of Dosage Units
[0068] Another embodiment of the present invention provides a dosage unit for inhibiting antral duodenal motility in a patient, which comprises GLP-1 and a pharmaceutically suitable excipient. The dosage unit is preferably about 0.4 to 2.4 pmol·kg -1 min -1 range between. And the dosage unit is preferably about 0.8 to 1.2 pmol·kg -1 min -1 range between. In the present invention, the term about is defined as ±10%. For example, the dosage unit is about 0.4 to 2.4 pmol·kg -1 min -1 range, that is, between 0.196 and 2.64pmol·kg -1 min -1 between.
[0069] The compositions of the present invention can be administered orally or parenterally for systemic or topical use. In addition, the composition can also be administered by intravenous infusion or subcutaneous injection. For the convenience of doctors or patients, the composition can be made into a dosage unit form containing a certain amount of GLP-1 molecules, for example, GLP-1...
Embodiment 1
[0099] Example 1 Motility Records and Blood Samples Obtained from Patients
[0100] A total of 11 healthy male volunteers participated in this study, aged from 23 to 28, and all within 10% of their ideal body weight. None of them were taking medication or suffering from gastrointestinal symptoms or systemic diseases.
[0101] All studies were performed after an overnight fast. There was at least one week between the two experiments, and the subjects took the semi-recumbent position during the experiment. An indwelling catheter is inserted in the antecubital vein for IV infusion. Arterialized venous blood was withdrawn by a second catheter retrogradely inserted in the dorsal vein of the contralateral hand [Schirra et al., Proc. Assoc. Am. Physicians, 109: pp. 84-97, (1997)].
[0102] Each experiment was preceded by a base period of at least 30 minutes, the last 15 minutes of which showed lower motor activity (less than 5 contractions per 10 minutes). During the interdiges...
Embodiment 2
[0104] Example 2 Motility recording of gastric antrum pyloric duodenal area
[0105] Perfusion pressures were recorded with a nine-lumen duodenal sleeve / side port catheter (Dentsleev, South Australia, Australia). The manometry device consisted of a 4.5 cm long sleeve sensor, two gastric antrum side holes (2 cm apart), and three duodenal side holes (2 cm apart), starting from the proximal and distal ends of the sleeve, respectively. Set two more side holes 1.5cm apart in the sleeve. An additional lumen was set 12cm away from the sleeve sensor for duodenal perfusion.
[0106] Duodenal probes with transpyloric cuff arrays were examined by fluoroscopy before each experiment as described in previous studies [Schirra et al., J. Clin Invest, 97:92-103, (1996)]. The correct position of the ostium was monitored throughout each study by measuring the transmucosal potential difference between the distal gastric antral ostium and the proximal duodenal ostium. A potential difference o...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com