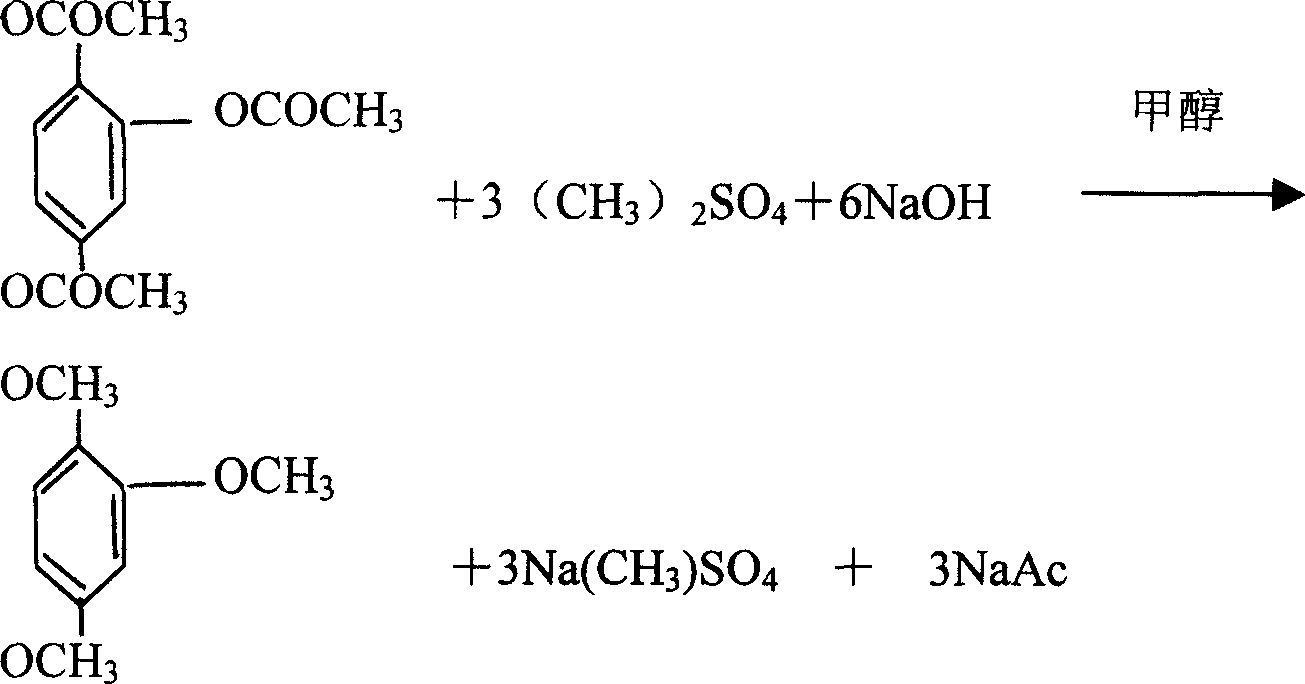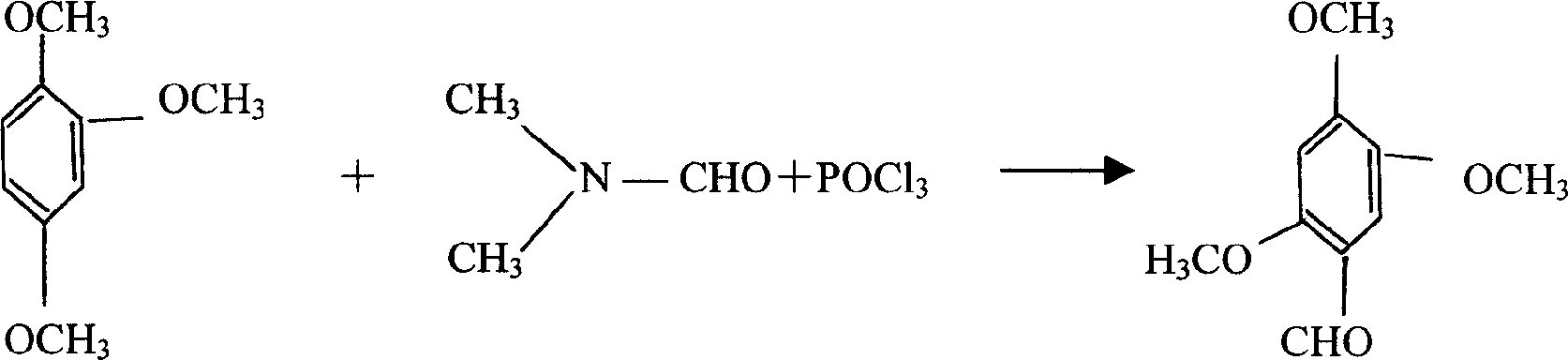Process for producing alpha-asarone raw material
A production process, the technology of asarone, applied in ether preparation, organic chemistry, etc., can solve the problems of low content of α-asarone, carcinogen safrole, and high extraction cost, so as to achieve excellent quality, increase yield, and reduce cost effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
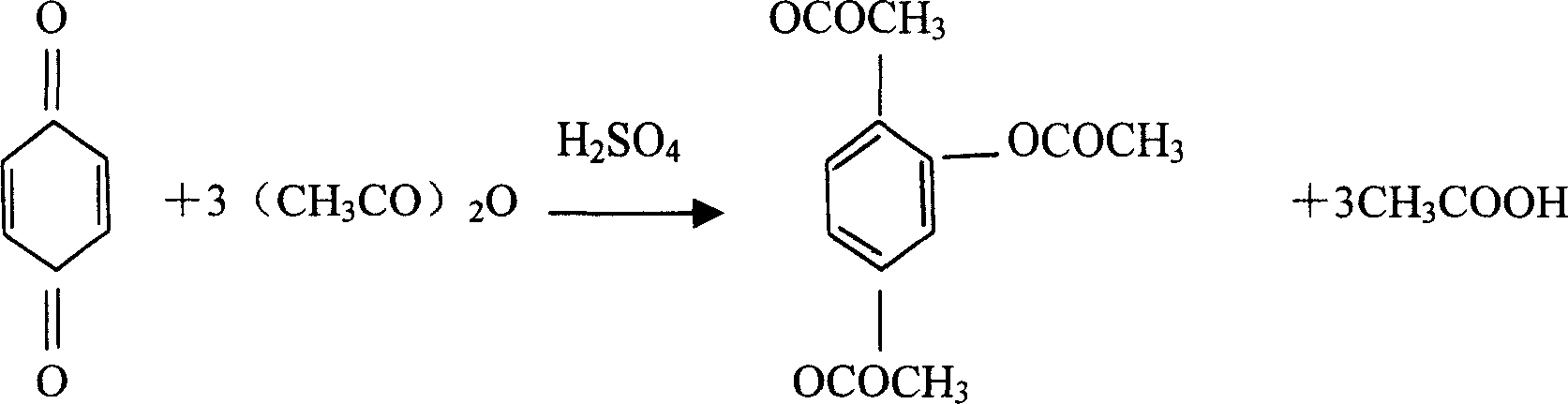
[0029] Concrete production technique of the present invention comprises the following stages:
[0030] 1. Acetylation reaction stage
[0031] raw material name
Specification
Proportion amount(kg)
Content ≥ 89%
1
Content ≥ 94%
3
Content≥97%
0.2
water
8
[0032] (2), the technological process of acetylation reaction is as follows:
[0033] Brush and dry the 300L enamel reaction tank, check that the valve at the bottom of the tank is closed, vacuum pump in an accurate amount of 90kg of acetic anhydride, add 3.3L of sulfuric acid while stirring, adjust the temperature of the material in the tank to 40°C or 42°C, and start adding For p-benzoquinone in the ratio of raw materials, the feeding speed should be uniform and uninterrupted, and the temperature should be controlled at 45°C. After the addition, the temperature should be natu...
Embodiment 2
[0064] The technological process of methylation reaction can also use following method, and other stages are with embodiment one:
[0065] Pump quantitative water into a 500L enamel reaction kettle, add 1.2.4-triacetoxybenzene that meets the ratio of raw materials for methylation reaction, stir for 10 minutes, add liquid caustic soda that meets the ratio of raw materials for methylation reaction, and then drop Add dimethyl sulfate that meets the ratio of raw materials for the methylation reaction, adjust the pH value at 8 or 9 after dripping for 2 hours, carry out extraction, let stand to separate layers, separate the oil layer, extract the water layer again, combine the oil layers and add The extraction layer was washed with water until neutral, the water layer was discarded, the solvent was recovered, and distillation was continued, the fraction was collected at 145°C, and the vacuum was 0.1MPA to obtain a yellow or light yellow transparent liquid, namely 1.2.4-trimethoxybenz...
Embodiment 3
[0067] The technological process of methylation reaction can also use following method, and other stages are with embodiment one:
[0068] Pump a certain amount of water into a 500L enamel reaction kettle, add 1.2.4-triacetoxybenzene that meets the ratio of raw materials for the methylation reaction, stir for 10 minutes, add liquid caustic soda dropwise, add dimethyl sulfate dropwise, Then dropwise add liquid caustic soda, then dropwise add dimethyl sulfate, then dropwise add liquid caustic soda, liquid caustic soda and dimethyl sulfate that meet the raw material ratio of the methylation reaction are alternately added dropwise until the reaction is complete, and a stable reaction temperature is always maintained. Stable PH value state, mature reaction for 2 hours, extraction, standing to separate layers, separate the oil layer, extract the water layer again, combine the oil layer and the extraction layer, wash with water until neutral, discard the water layer, recover the solve...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com