Method for raising SPE hamster for preparing various biological products
A technology of biological products and pathogens, which is applied in the fields of vehicle rescue, urns, animal husbandry, etc., and can solve the problems that the safety of biological products is not guaranteed.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
reference example 1
[0048] Microbial Contamination Experiment
[0049] 1-1) Detection of parasites
[0050] After tape-taking the specimen, examine it with a light microscope for the presence of any exogenous parasites. At the same time, to check for the presence of any endogenous parasites, the experimental animals were sacrificed, dissected, and stomach, small intestine, and large intestine tissue samples were prepared. The specimens were stained with hematoxylin and eosin and examined under a light microscope.
[0051] 1-2) Detection of bacteria
[0052] Check for the presence of bacteria based on the following three methods.
[0053] a. Culture method—for all bacteria except Helicobacter spirochetes, Mycoplasma pneumoniae, and Clostridioides.
[0054] Tracheal, sinus, and intestinal material was taken with sterile cotton swabs and spread out on culture plates for bacterial testing. Then, follow the SOP (Standard Operating Procedure, standard operating procedure) for processing.
[0...
reference example 2
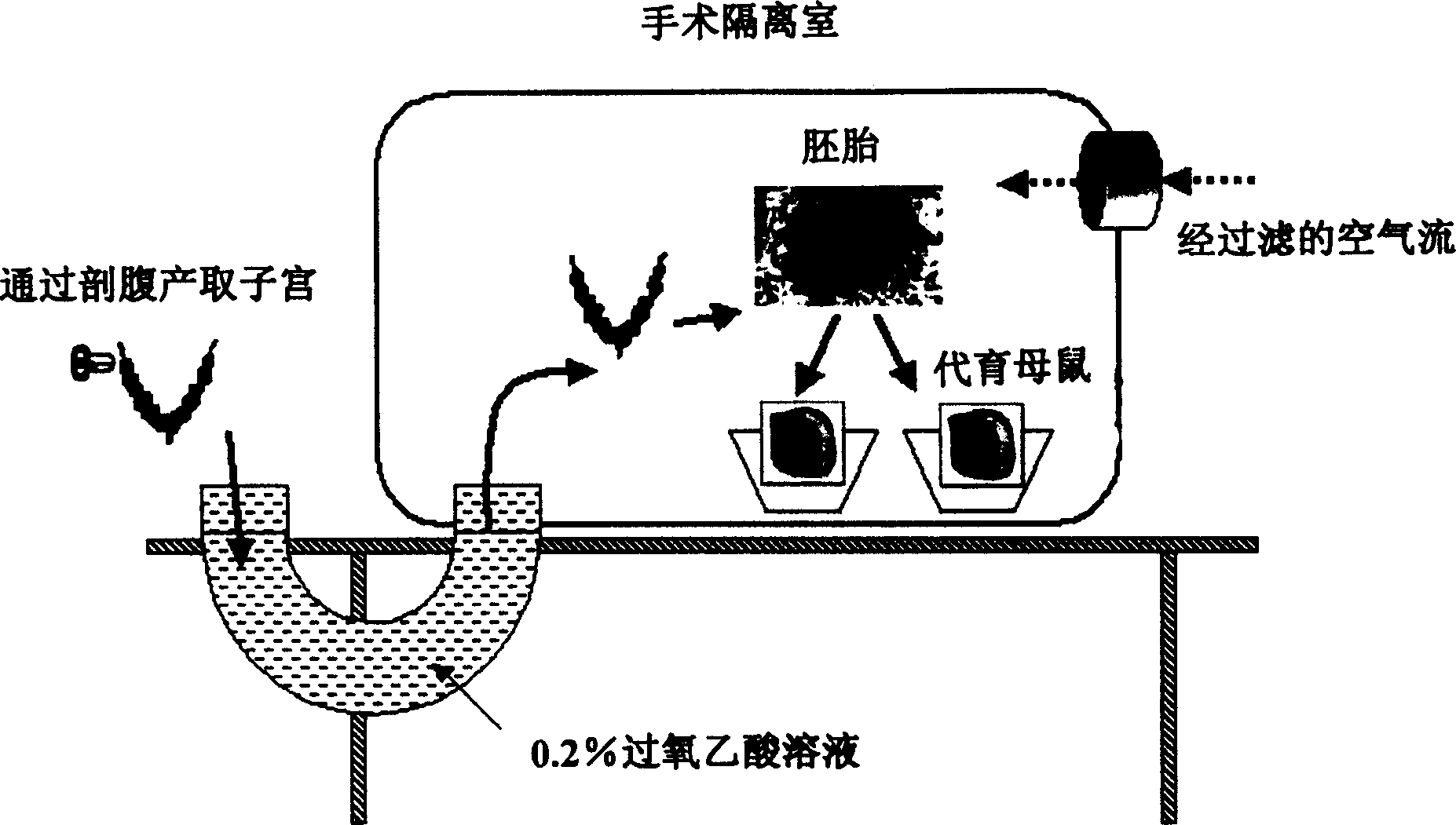
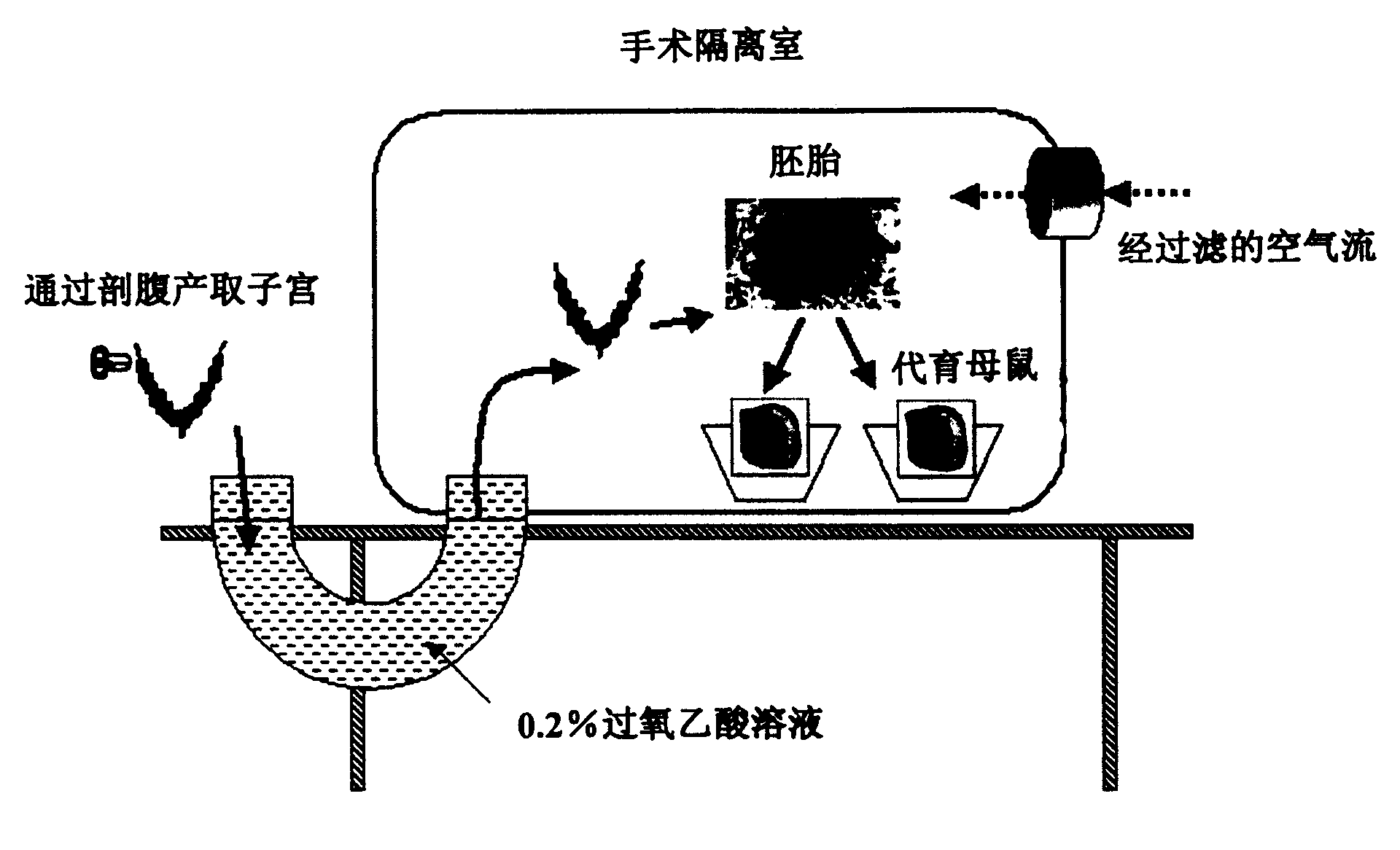
[0062] Preparation of the Surgical Isolation Room
[0063] The interior of a surgical isolation room (Daehan Biolink Co. Ltd., Korea) was sterilized with alcohol and chlorhexidine gluconate solution, and then its positive pressure was maintained with active air flow. A immersion bath was filled with a 0.2% peracetic acid solution. The operating tools and other necessities are put into this isolation room for the surgical operation, and a blood agar plate (SIGMA) is prepared for immediate detection of possible microbial contamination in the specimen (freshly taken from the embryo).
[0064]
[0065] Screening of hamsters for production of SPF hamsters
[0066] 1-1) Initial screening of the hamster group
[0067] Following the method described in Reference Example 1, the golden Syrian hamsters obtained from the Chengdu Institute of Biological Products in China were subjected to microbial contamination experiments (see Table 1). As a result, a group of hamsters not conta...
experiment example 1
[0137] Safety test of Japanese hepatitis attenuated live vaccine in the present invention
[0138] In order to confirm the safety of the Japanese hepatitis live attenuated vaccine on the human body, a safety test was carried out. The Japanese hepatitis live attenuated vaccine was prepared with the PHK cells of the SPF hamster (Example 5) of the present invention.
[0139] 1-1) Detection of HPyV
[0140] In order to detect whether there is HPyV in the Japanese hepatitis attenuated live vaccine of the present invention, use ABI 7700 sequence detection system to determine whether there is HPyV specific sequence, the method is as described in Example 5-1). The results are summarized in Table 13 below.
[0141] test group
HPyV-specific sequence
negative control group
* -
Japanese hepatitis attenuated live vaccine of the present invention
-
positive control group
* +
[0142] * -: not detected
[0143] *...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com