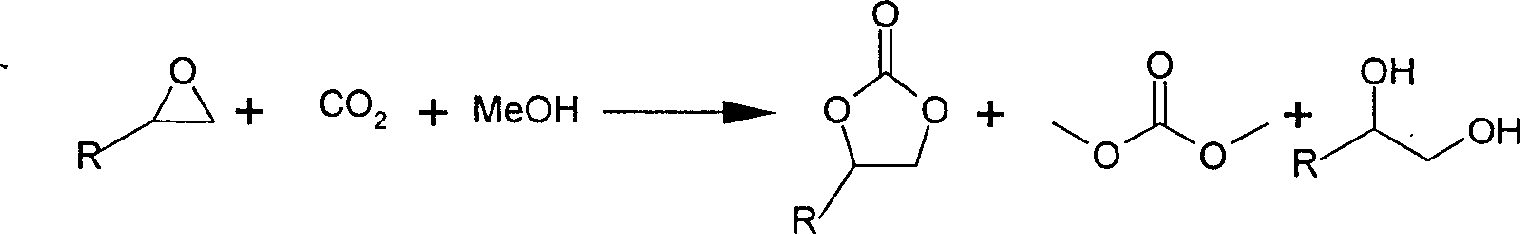
Direct synthesis process of methyl carbonate
A dimethyl carbonate, direct technology, applied in carbon dioxide or inorganic carbonate preparation, bulk chemical production, organic chemistry, etc., can solve the problems of poor selectivity and low yield, and achieve high yield and good selectivity Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0017] Add 0.44g (10mmol) of oxirane in reaction vessel, methanol 1.28g (40mmol) and carbon dioxide 6.1g (139mmol), add the catalyst 0.25g of 3.19wt% of reaction mixture weight, catalyst is KI 30wt%, ZnO55wt% and K 2 CO 3 15% by weight of the composition. Stir, heat up to 150°C, and react for 2 hours. The temperature was lowered to 120° C., and the reaction was continued for 2 hours. After the reaction was finished, it was cooled, filtered, and the catalyst was removed, and the filtered reaction solution was analyzed by gas chromatography. The conversion rate of ethylene oxide was 99.3%, and the selectivity of dimethyl carbonate was 70.8%.
Embodiment 2
[0019] In reaction vessel, add propylene oxide 0.58g (10mmol), methyl alcohol 1.92g (60mmol) and carbon dioxide 8.8g (200mmol), add the catalyzer 0.23g of 2.03wt% of reaction mixture weight, catalyzer is KCl 45wt%, ZrO40wt% and Na 2 CO 3 15% by weight of the composition. Stir, heat up to 160°C, and react for 4 hours. The temperature was lowered to 130° C., and the reaction was continued for 4 hours. After the reaction, supercritical CO 2 Extraction, the obtained reaction solution was analyzed by gas chromatography, the conversion rate of propylene oxide was 98.2%, and the selectivity of dimethyl carbonate was 41.5%.
Embodiment 3
[0021] Add phenyloxirane 0.72g (6mmol) in reaction vessel, methyl alcohol 1.92g (60mmol) and carbon dioxide 5.3g (120mmol), add the catalyzer 0.32g of 4.03wt% of reaction mixture weight, catalyzer is KBr 25wt%, Composition of NaX type molecular sieve 60wt% and NaOH 15wt%. Stir, heat up to 170°C, and react for 3 hours. The temperature was lowered to 110° C., and the reaction was continued for 6 hours. After the reaction, supercritical CO 2 Extraction, the obtained reaction liquid was analyzed by gas chromatography, the conversion rate of phenyloxirane was 98.5%, and the yield rate of dimethyl carbonate was 54.8%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com