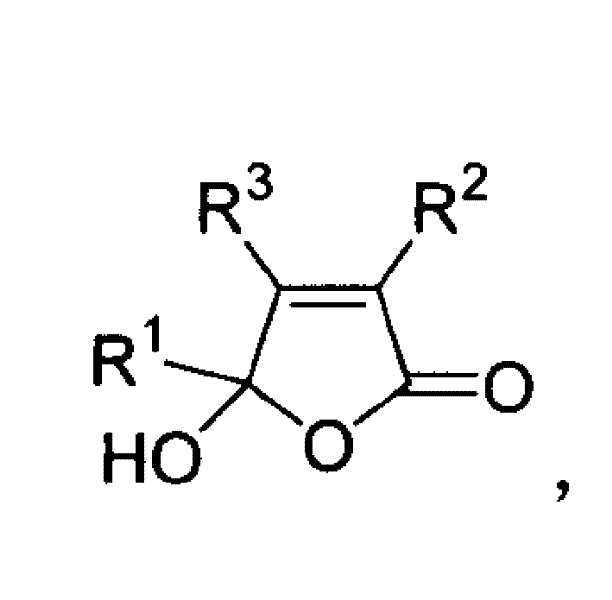
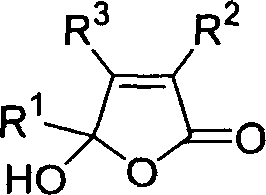
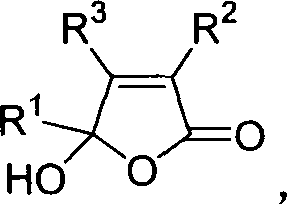
3,4,5-trisubstituted-5-hydroxy-2(5hydro)-furaldone compound and its synthesis and use
A synthetic method and technology of furanone are applied in 3 fields to achieve the effects of convenient post-processing, simple reaction equipment and high reaction yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025]
[0026] (1) 3-methyl-4-iodo-5-phenyl-5-hydroxy-2(5hydrogen)-furanone:
[0027] Take a reaction tube, add 2-methyl-4-phenyl-2,3-butadienoic acid (86mg, 0.494mmol) in 2mL THF solution, add LiOAc·2H 2 O (63mg, 0.62mmol) and I 2 (250mg, 0.98mmol), stirred at room temperature for 3 hours, added 1ml of DMF to the reaction system, protected the reaction system with an oxygen balloon at 1 atmosphere, heated the oil bath at 40°C for 36 hours, extracted the reaction solution with ether, and washed it with 5% Na 2 S 2 o 3 washed with brine, anhydrous Na 2 SO 4 Drying, column chromatography (petroleum ether: ethyl ether = 3.5: 1) purified to obtain 136mg of 3-methyl-4-iodo-5-phenyl-5-hydroxyl-2(5hydrogen)-furanone, the yield 87%. Solid, melting point 150-152°C (acetone / n-hexane); 1 H NMR (300MHz, CD 3 COCD 3 )δ7.60-7.38 (m, 5H), 7.25-7.15 (bs, 1H), 1.94 (s, 3H); 13 C NMR (75.4MHz, CD 3 COCD 3 )δ169.9, 137.9, 137.1, 130.2, 129.3, 127.3, 127.2, 107.2, 13.2; MS (m / z) 3...
Embodiment 2
[0029]
[0030] (2) 3-n-propyl-4-iodo-5-phenyl-5-hydroxy-2(5hydrogen)-furanone:
[0031] Take a reaction tube, add 2-n-propyl-4-phenyl-2,3-butadienoic acid (101 mg, 0.50 mmol) in 2 mL THF solution, add LiOAc·2H 2 O (65mg, 0.64mmol) and I 2 (257mg, 1.01mmol), stirred at room temperature for 3 hours, added 1ml of DMF to the reaction system, protected the reaction system with an oxygen balloon at 1 atmosphere, heated the oil bath at 40°C for 34 hours, extracted the reaction solution with ether, and washed it with 5% Na 2 S 2 o 3 washed with brine, anhydrous Na 2 SO 4 Drying, column chromatography (petroleum ether: ether = 3.5: 1) purified to obtain 143mg of 3-n-propyl-4-iodo-5-phenyl-5-hydroxyl-2(5hydrogen)-furanone, producing The rate is 83%. liquid; 1 H NMR (300MHz, CD 3 COCD 3 )δ7.62-7.35(m, 5H), 7.22(s, 1H), 2.34(t, J=7.7Hz, 2H), 1.73-1.52(m, 2H), 0.96(t, J=7.4Hz, 3H ); 13 C NMR (75.4MHz, CD 3 COCD 3 )δ169.0, 139.6, 137.3, 129.5, 128.6, 127.3, 126.5, 106.4, 29...
Embodiment 3
[0033]
[0034] (3) 3-benzyl-4-iodo-5-phenyl-5-hydroxy-2(5hydrogen)-furanone:
[0035] Take a reaction tube, add 2-benzyl-4-phenyl-2,3-butadienoic acid (100mg, 0.40mmol) in 2mL THF solution, add LiOAc·2H 2 O (55mg, 0.54mmol) and I 2 (124mg, 0.49mmol), stirred at room temperature for 3 hours, added 1ml of DMF to the reaction system, protected the reaction system with an oxygen balloon at 1 atmosphere, heated the oil bath at 40°C for 8 hours, extracted the reaction solution with ether, and washed it with 5% Na 2 S 2 o 3 washed with brine, anhydrous Na 2 SO 4 Drying, column chromatography (petroleum ether: ether = 3.5: 1) purified to obtain 113mg of 3-benzyl-4-iodo-5-phenyl-5-hydroxyl-2(5hydrogen)-furanone, yield 72%. Solid, melting point 151-155°C (acetone / n-hexane); 1 H NMR (300MHz, CD 3 COCD 3 )δ7.60-7.18 (m, 10H), 3.72 (s, 2H), 2.86 (s, 1H); 13 C NMR (75.4MHz, CD 3 COCD 3 )δ170.0, 139.9, 138.4, 138.1, 130.7, 130.1, 129.9, 129.7, 129.4, 128.2, 127.6, 107.8, 34.3...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com