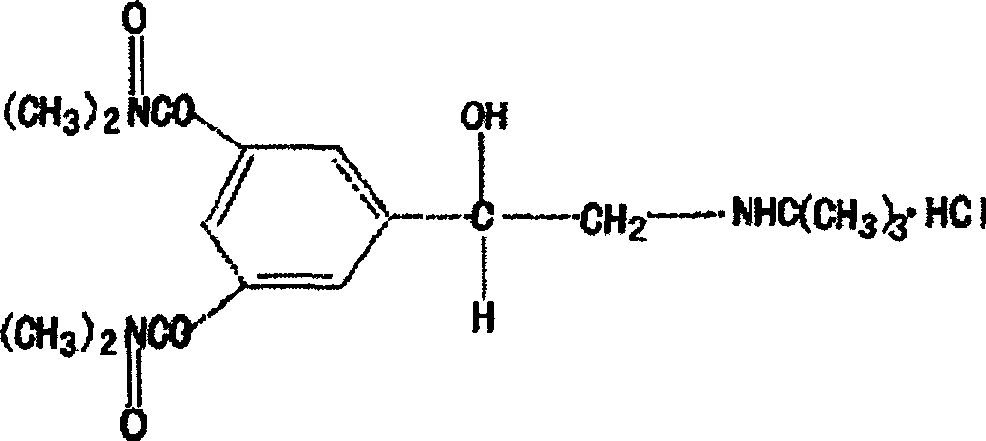
Oral disintegrating tablet of bambuterol hydrochloride and its preparation method
A technology of bambuterol hydrochloride and orally disintegrating tablets, which is applied in the fields of pill delivery, respiratory system diseases, and active ingredients of esters. Good therapeutic effect and disintegration performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] The orally disintegrating tablet of bambuterol hydrochloride includes main ingredients and auxiliary materials, and is characterized in that it is prepared according to the following weight percentages: 5% of main ingredients and 95% of auxiliary materials.
[0043] The orally disintegrating tablet of the present invention is prepared from the following raw materials in weight ratio:
[0044] Bambuterol Hydrochloride 5.0g
[0045] Lactose 44.0g
[0046] Mannitol 17.5g
[0047] Microcrystalline Cellulose 21.6g
[0048] Low-substituted hydroxypropyl cellulose 2.4g
[0049] Cross-linked polyvinylpyrrolidone 5.0g
[0050] Sodium bicarbonate 1.0g
[0051] Citric acid 1.0g
[0052] Aspartame 0.5g
[0053] Mint essence 0.25g
[0054] Cherry essence 0.75g
[0055] Magnesium Stearate 1.0g
[0056] A total of 1000 tablets were produced
[0057] Among them, lactose and mannitol are fillers. Low-substituted hydroxypropyl cellulose, microcrystalline cellulose and cross-l...
Embodiment 2
[0067] The orally disintegrating tablet of bambuterol hydrochloride includes main ingredients and auxiliary materials, and is characterized in that it is prepared according to the following weight percentages: 10% of main ingredients and 90% of auxiliary materials.
[0068] The orally disintegrating tablet of the present invention is prepared from the following raw materials in weight ratio:
[0069] Bambuterol Hydrochloride 10.0g
[0070] Lactose 40.0g
[0071] Mannitol 20.0g
[0072] Microcrystalline Cellulose 20.0g
[0073] Low-substituted hydroxypropyl cellulose 4.0g
[0074] Cross-linked polyvinylpyrrolidone 5.0g
[0075] Sodium bicarbonate 1.0g
[0076] Citric acid 1.0g
[0077] Aspartame 0.5g
[0078] Mint essence 0.25g
[0079] Cherry essence 0.75g
[0080] Magnesium Stearate 1.0g
[0081] A total of 1000 tablets were produced
[0082] Among them, lactose and mannitol are fillers. Low-substituted hydroxypropyl cellulose, microcrystalline cellulose and cross...
Embodiment 3
[0094] The orally disintegrating tablet of bambuterol hydrochloride includes main ingredients and auxiliary materials, and is characterized in that it is formulated according to the following weight percentages: 20% of main ingredients and 80% of auxiliary materials.
[0095] The orally disintegrating tablet of the present invention is prepared from the following raw materials in weight ratio:
[0096] Bambuterol Hydrochloride 20.0g
[0097] Lactose 10.0g
[0098] Mannitol 27.0g
[0099] Microcrystalline Cellulose 15.0g
[0100] Low-substituted hydroxypropyl cellulose 10.0g
[0101] Cross-linked polyvinylpyrrolidone 3.0g
[0102] Sodium bicarbonate 5.0g
[0103] Citric acid 5.0g
[0104] Aspartame 1.0g
[0105] Mint essence 0.15g
[0106] Cherry essence 0.85g
[0107] Magnesium stearate 0.5g
[0108] A total of 1000 tablets were produced
[0109] Among them, lactose and mannitol are fillers. Low-substituted hydroxypropyl cellulose, microcrystalline cellulose and cr...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Hardness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com