Prepn of high-content vitamin E
A vitamin and high-content technology, applied in the direction of organic chemistry, can solve the problems of high cost, complicated process, high content, etc., and achieve the effect of low cost and simple process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0016] Embodiment 1: (existing method, promptly does not add procatalyst of the present invention)
[0017] At room temperature, in a 500ml three-necked flask, 51.1 grams (331mmol) of trimethylhydroquinone and an equivalent amount of zinc chloride and 4.7ml of concentrated hydrochloric acid (0.05mmol) were mixed with 150ml of ethyl acetate, and the solution was heated to 40°C, add 100g (330mmol) isophytol dropwise within 2 hours, keep the reaction mixture at 40°C for 1.5 hours, then raise the temperature to 60-65°C, keep it warm for 2 hours, depressurize the reaction solution to recover ethyl acetate Ester, the obtained crude product is esterified with acetic anhydride, and then the content of vitamin E in the product obtained by rectification is 96.0%, and the content of dl-α-tocopherol isomers is 2.5%.
Embodiment 2
[0018] Embodiment 2: (the present invention)
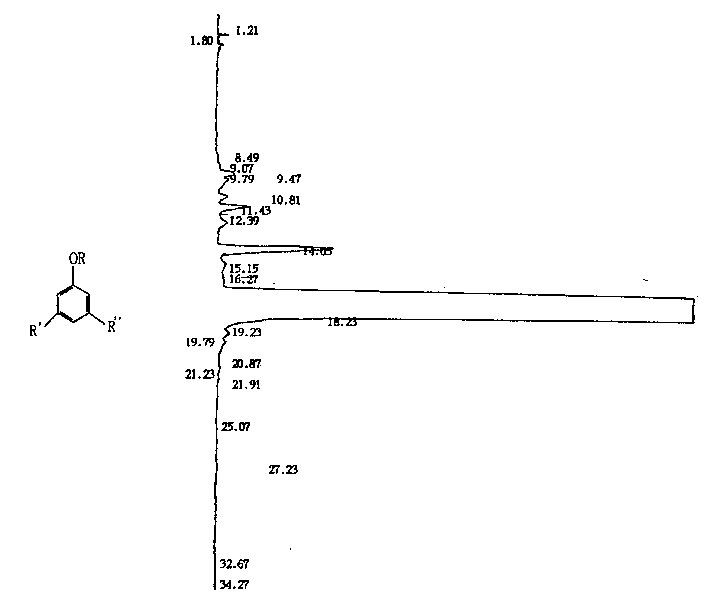
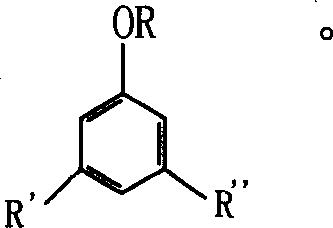
[0019] At room temperature, in a 500ml three-necked flask, 51.1 grams (331mmol) of trimethylhydroquinone and an equivalent amount of zinc chloride and 4.7ml of concentrated hydrochloric acid (0.05mmol) were mixed with 150ml of ethyl acetate, and 1.5 grams (for 1.5% of isophytol weight) 1,3,5-trimethylbenzene, structural formula is The amount of isophytol added and the preparation process and process conditions are the same as those in Example 1 to obtain a vitamin E product with a content of 99.3%, and the content of dl-α-tocopherol isomers in the product is 0.29%.
[0020] The difference between Examples 3-10 and Example 2 lies in the cocatalyst added, and the obtained data are listed in Table 1.
[0021] The difference between Examples 11-16 and Example 2 lies in the amount of co-catalyst added, and the obtained data are listed in Table 2.
[0022] Table 1: select the data table of cocatalyst gain of the pres...
Embodiment 5
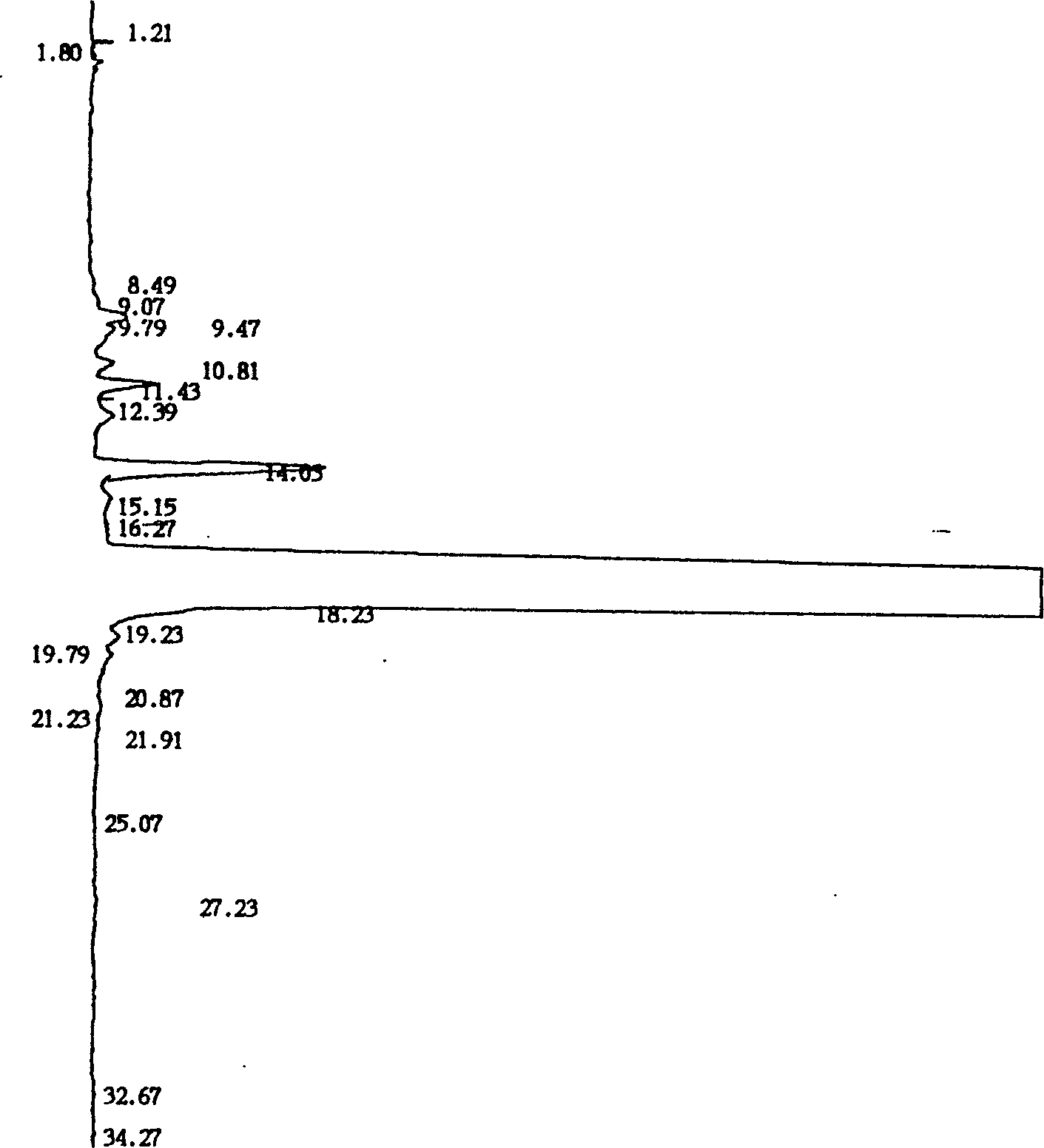
[0028] The gas chromatogram data list in embodiment 5 is as follows:
[0029] Peak width (WD): 3 Slope (SL): 50.00 Calculation method: area normalization method
[0030] Peak No. Component Name Component No. Retention Time Peak Area Peak Height Content Peak Marker
[0031] 1 0 1.21 98.0 56 0.0033 S
[0032] 2 0 1.80 137.8 23 0.0046 S
[0033] 3 0 8.49 90.6 4 0.0030 S
[0034] 4 0 9.07 211.6 13 0.0071 F
[0035] 5 0 9.47 1601.6 82 0.0539 M
[0036] 6 0 9.79 1288.1 51 0.0433 M
[0037] 7 0 10.81 803.1 44 0.0270 M
[0038] 8 0 11.43 2347.3 141 0.0790M
[0039] 9 0 12.39 1243.3 43 0.0418 M
[0040] 10 0 14.03 12373.5 531 0.4162 S
[0041] 11 0 15.15 1284.7 34 0.0432 M
[0042] 12 0 16.27 901.3 28 0.0303 F
[0043] 13 0 18.23 2946150.8 56382 99.1055 M
[0044] 14 0 19.23 1465.6 55 0.0493 M
[0045] 15 0 19.79 1370.6 38 0.0461 M
[0046] 16 0 20.87 47.3 8 0.0016 M
[0047]17 0 21.23 358.8 13 0.0121 M
[0048] 18 0 21.91 152.3 7 0.0051 M
[0049] 19 0 25.07 32.1 1 0....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com