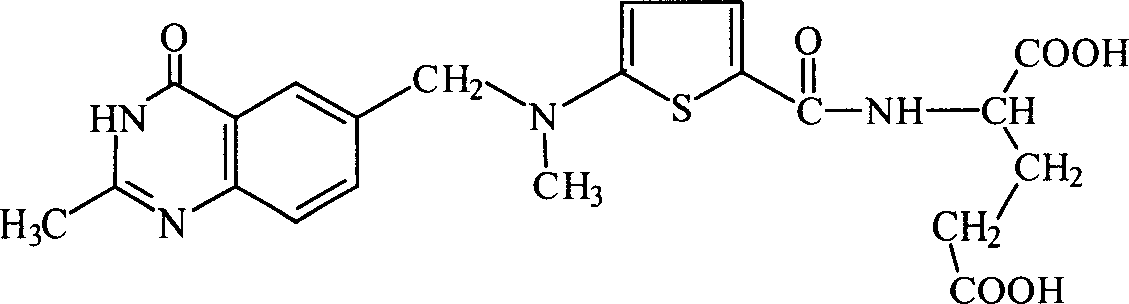
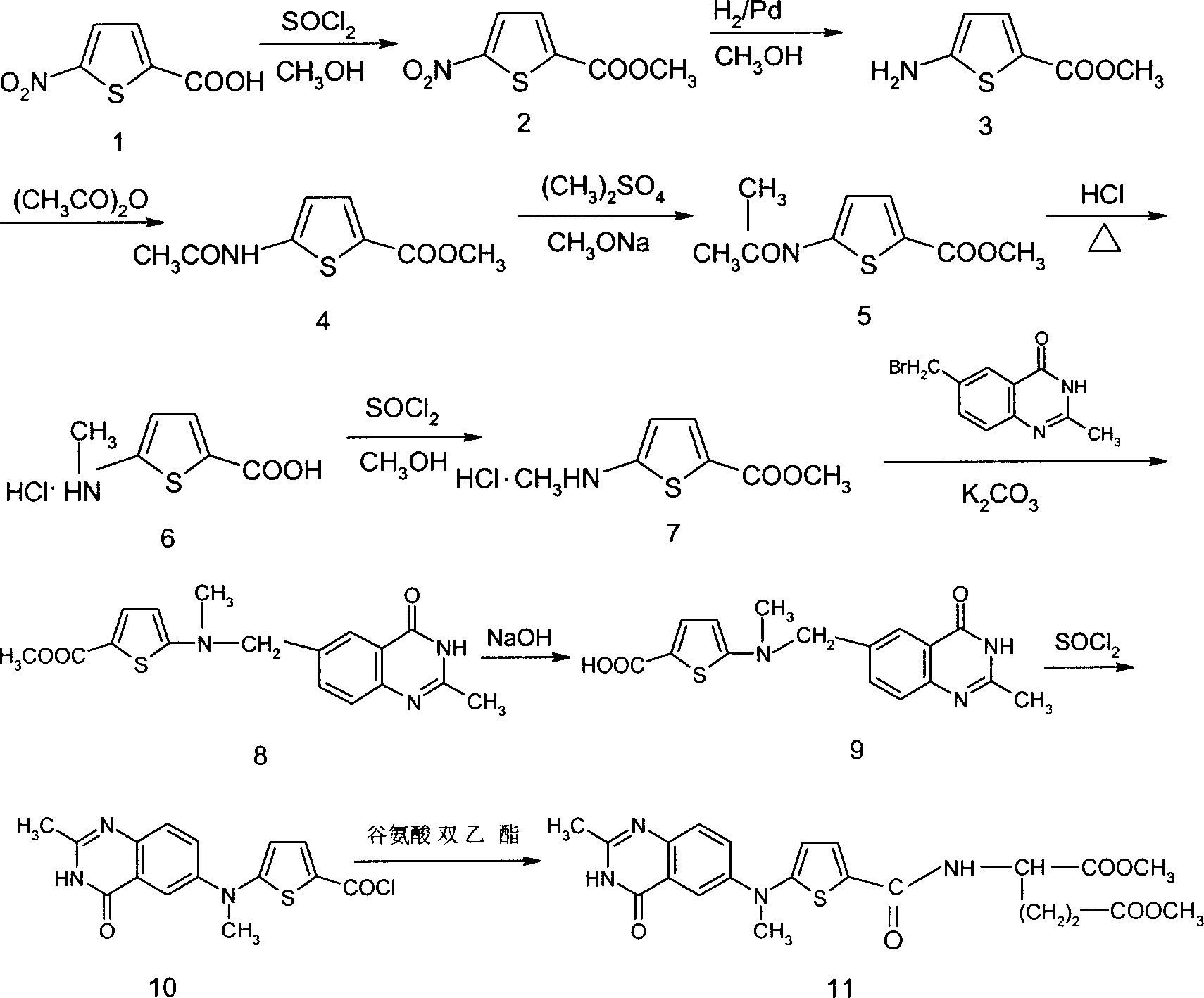
Preparation of Leiliqusai
A preparation process and technology of raltitrexed, applied in the field of medicine, can solve the problems of low yield, harsh reaction conditions, affecting the popularization and application of raltitrexed, etc., and achieves reduction of production cost, mild reaction conditions, and improved synthesis yield. rate and quality of finished products
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
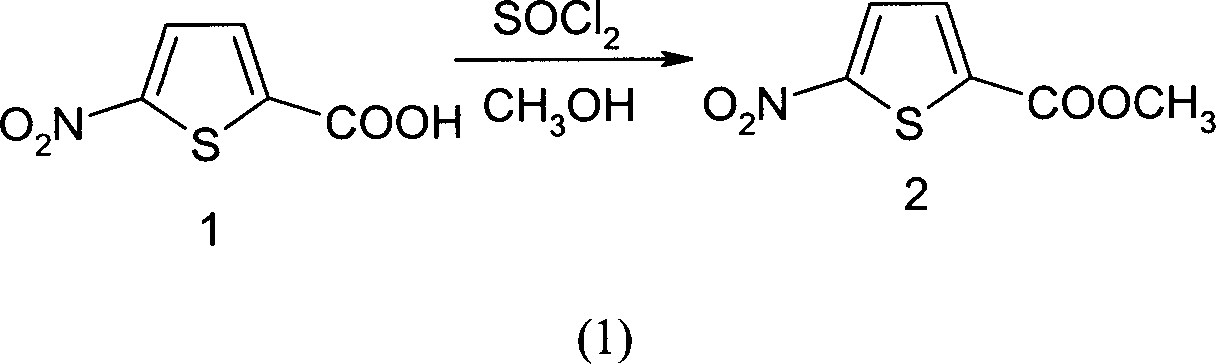
[0017] Preparation of intermediate 2 (methyl 5-nitro-thiophene-2-carboxylate): under ice-salt cooling, 173g Add to 1000ml of methanol, drop 75ml of SOCl at less than -10°C 2 After the addition, the reaction was carried out overnight at room temperature, the solvent was evaporated to dryness, and the intermediate 2 was obtained by distillation under reduced pressure with a yield of 91.7%. The reaction formula is as (1):
[0018]
example 2
[0020] Preparation of intermediate 3 (methyl 5-aminothiophene-2-carboxylate): dissolve the product obtained in Example 1 (intermediate 2) in 200 ml of methanol, add 1 g of 10% palladium carbon, catalytic hydrogenation, filter, and evaporate to dryness Solvent was used to obtain intermediate 3 with a yield of 89.3%. The reaction formula is as (2):
[0021]
example 3
[0023] Preparation of intermediate 4 (5-(N-acetyl-)-aminothiophene-2-carboxylic acid methyl ester): the product obtained in the above example 2 (intermediate 3) was dissolved in HOAc, and an equivalent amount of acetic anhydride was added dropwise, Stir at room temperature until the reaction of the raw materials is complete, and evaporate the solvent to obtain intermediate 4 with a yield of 87.9%. The reaction formula is as (3):
[0024]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com