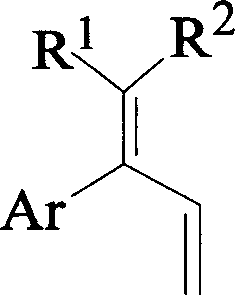
Synthesizing compound of 1, 1, 2 triaromatic radical-1, 3 butadiene kind by suzuki-miyaura coupling reaction
A technology for butadiene and compounds, which is applied in the field of synthesizing anti-breast cancer natural products, can solve the problems of complex operation, not easy operation, long route, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0073] The general operation steps of the reaction
[0074] Under argon protection, add diiodide 1 (0.25mmol), aryl boronic acid 2 (0.30mmol), catalyst Pd(PPh 3 ) 4 (0.025mmol), tetrabutylammonium chloride (TBAC) (0.25mmol) and base KOH (1.2mmol), pumped three times after the addition was complete. Cool the system to -78°C with a dry ice-acetone bath, then add tetrahydrofuran and water (1.0 mL / 0.3 mL), and ventilate three times. Heating, stirring, reflux, and maintain reflux reaction for 48 hours. The reaction solution was diluted with dichloromethane and dried over anhydrous sodium sulfate. Spin dry, flash column chromatography to get the corresponding product.
[0075] Product 4. Colorless liquid, 1 H NMR (CDCl 3 , 300MHz, TMS) δ5.33 (dd, 1H, J=0.9, 10.2Hz), 5.65 (dd, 1H, J=0.9, 15.6Hz), 6.13 (dd, 1H, J=10.2, 15.6Hz), 7.16 -7.37(m, 10H, Ar). 13 C NMR (CDCl 3 , 75MHz, TMS) δ105.91, 124.52, 127.61, 127.70, 128.05, 128.19, 129.11, 129.72, 136.84,...
Embodiment 2
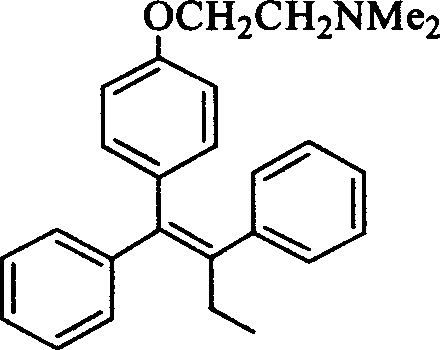
[0092] The shortest steps for the synthesis of (E,Z)-Tamoxifen from product 3ba:
[0093] Scheme 1 Synthesis of (E, Z)-Tamoxifen from 3ba.
[0094]
[0095] A. Restoration steps:
[0096] 3ba (0.44mmol) was dissolved in 99% ethanol (0.5mL) and dichloromethane (2.0mL), then added 85% hydrazine hydrate (3.6mmol), cooled to -60°C in a dry ice-acetone bath, and stirred within 10 minutes Additional 35% hydrogen peroxide (2.6 mmol) was added. After the addition was complete, the system was allowed to warm to room temperature naturally and stirred at room temperature for 24 hours. The reaction solution was diluted with dichloromethane, washed with saturated aqueous sodium sulfite, and extracted with dichloromethane. The organic phases were combined, washed with saturated brine, dried over sodium sulfate, and spin-dried under reduced pressure. Crude product A for 1 Confirmed by H NMR, it was directly used in the next reaction without further treatment.
[00...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com