DNA vaccine carrier of carring SV40 enhancer element
A technology of DNA vaccines and vectors, applied in the fields of biotechnology and genetic immunity, can solve problems such as the difficulty of inferring the impact of DNA vaccine immunogenicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Example 1: Construction of DNA vaccine vector pDRVISV1.0
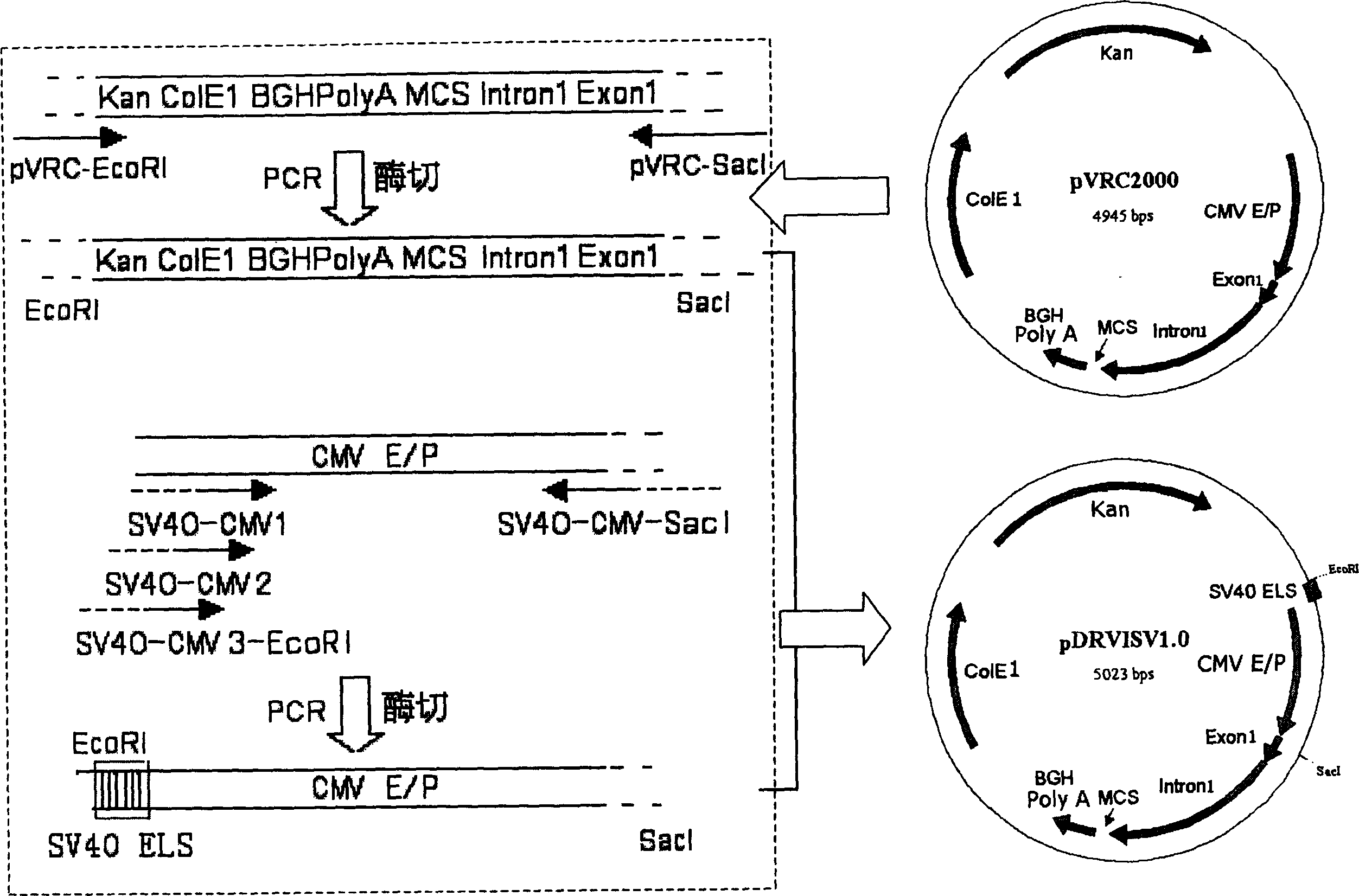
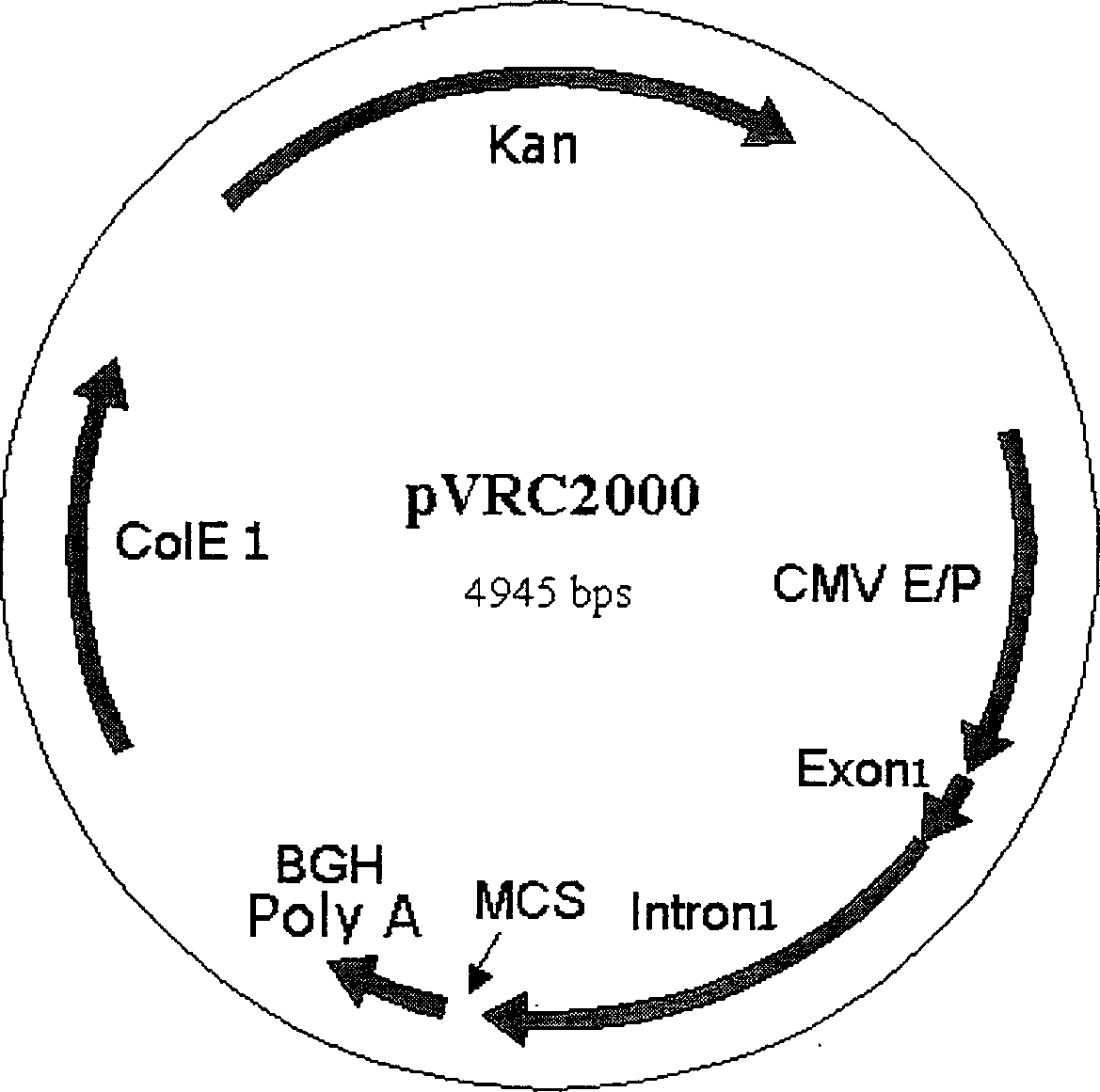
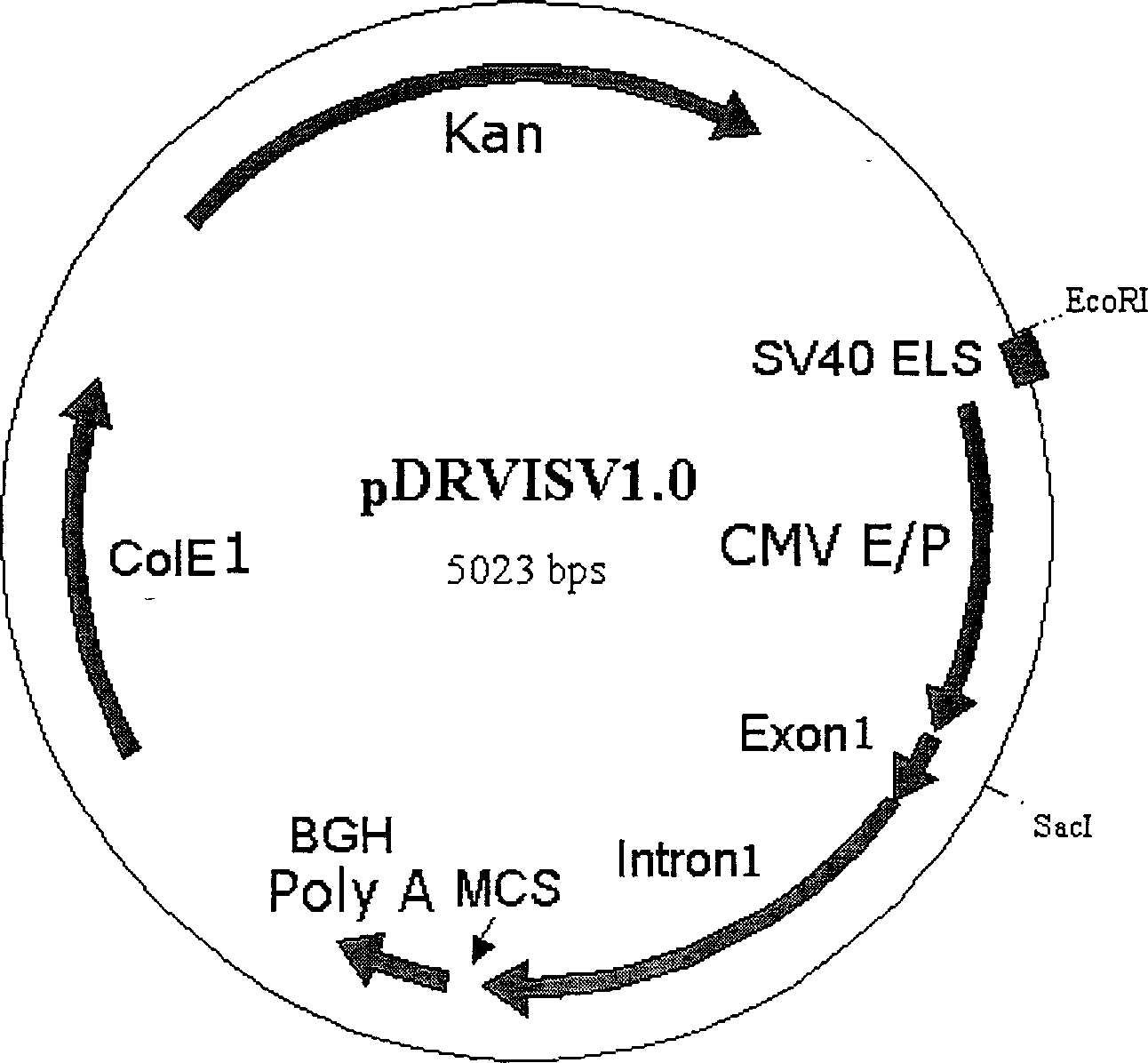
[0026] Using a combination of PCR technology and subcloning technology, a 72bp single-copy SV40 enhancer sequence was inserted into the upstream of the pVRC2000CMV promoter to construct plasmid pDRVISV1.0 (see figure 1 ). The specific construction process is as follows.
[0027] Using plasmid pVRC2000 as a template, primers 5'SV40-CMV1 (5'-ggagcctggggactttccacaccattaccgccatgttgacattg-3' (SEQ ID NO: 1)) and 3'SV40-CMV-SacI (5'-aacagtatagcatgcataagagccaaag-3' (SEQ ID NO: 2) )) PCR amplification was carried out, and the obtained PCR product was designated as SV40-CMV1. In this reaction, a 72bp single-copy SV40 enhancer partial sequence (ggagcctggggactttccacacc (SEQ ID NO: 3)) was actually introduced upstream of the CMV promoter, and the specific band was recovered by 1% agarose gel electrophoresis with a gel recovery kit DNA (about 750bp). The PCR reaction system is:
[0028] 10×Pyrobest Buffer 5μl ...
Embodiment 2
[0087] Example 2: Construction of recombinant DNA vaccine pVRC2000-gag and pDRVISV1.0-gag
[0088] Using gagwt5SalI (5'-acgcgtcgacgccgccaccatggccgccagggccagcatc-3' (SEQ ID NO: 13)) and gags3EcoRV (5'-acgtgatatctcactggctgctggggtcgttgcc-3' (SEQ ID NO: 14)) as primers, amplified from plasmid pCRScript-gpnef by PCR HIV-1 CN54gag gene (about 1.5kb), SalI and EcoRV double restriction restriction directional clone into DNA vaccine vectors pVRC2000 and pDRVISV1.0, to obtain recombinant DNA vaccines pVRC2000-gag and pDRVISV1.0-gag, the construction process see Figure 4 .
[0089] The PCR reaction system is:
[0090] 10×Pyrobest Buffer 5μl
[0091] dNTP mix (2.5mM each) 5μl
[0092] gagwt5SalI (50 μM) 0.5 μl
[0093] gags3EcoRV (50μM) 0.5μl
[0094] pCRScript-gpnef 0.5μl
[0095] Pyrobest DNA Polymerase (5U / ml) 0.5μl
[0096] wxya 2 O 38 μl
[0097] The reaction conditions were: pre-denaturation at 94°C for 5 min; ...
Embodiment 3
[0113] Example 3: Comparison of humoral and cellular immune responses induced after immunization with pVRC2000-gag and pDRVISV1.0-gag
[0114] Prepare DNA vaccine pVRC2000-gag, pDRVISV1.0-gag and empty DNA vaccine vector plasmid DNA with Qiagen Giga Endo-Free Prep kit, dissolve them in saline respectively, and make a plasmid solution with a concentration of 1g / l. There were 5 mice in each experimental group (n=5), and 5 mice in each of the empty vector control and the blank control. The tibialis anterior muscle of both hind legs was injected, DNA vaccine immunization group and vector control group were injected with 100g / 100l plasmid DNA, and the blank control group was injected with 100l normal saline. After the initial immunization, the same dose was boosted 3 times at intervals of 2 weeks. At the 8th week, orbital blood was collected to obtain serum, and then sacrificed by dismounting the cervical spine, and the spleen was taken to make a single cell suspension (6×10 5 ce...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com