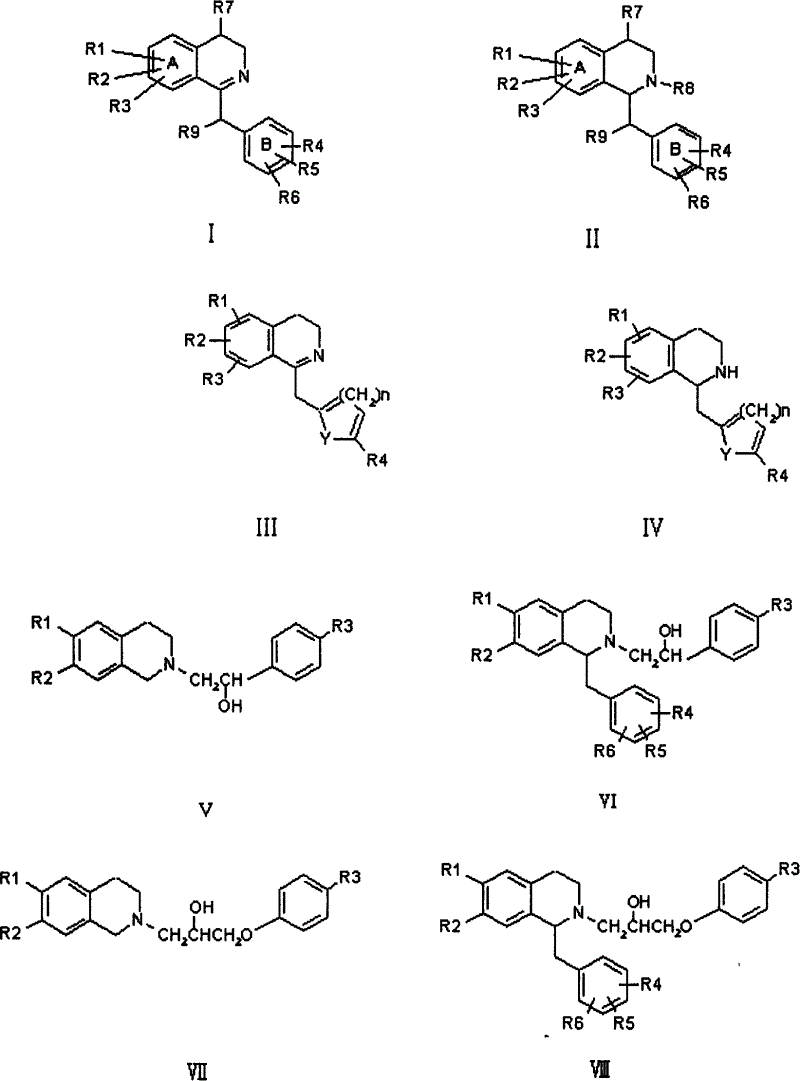
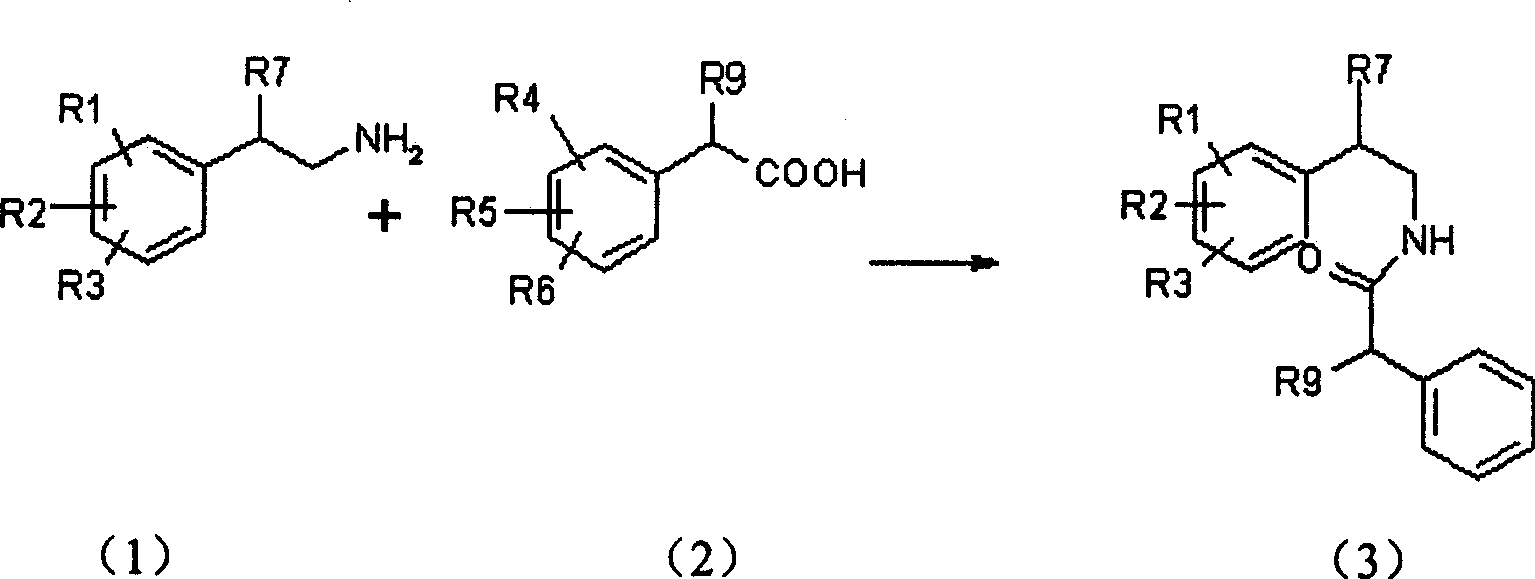
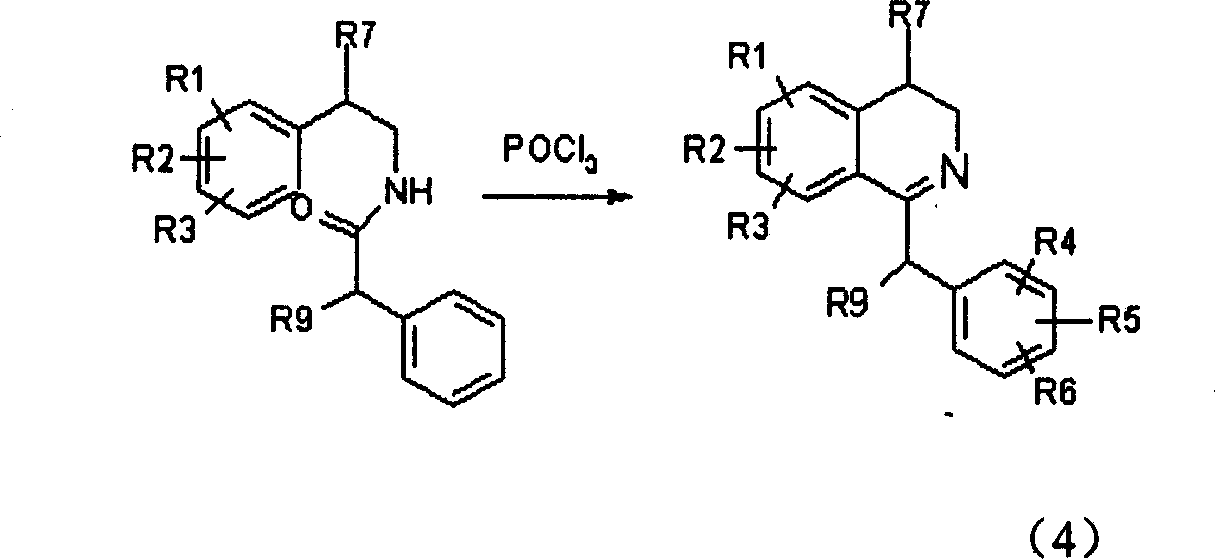
Isoquinoline compound, preparation method and application of salt thereof
A technology of tetrahydroisoquinoline hydrochloride and compounds, applied in the field of preparation of antiarrhythmic drugs, capable of solving problems such as poor oral absorption and irregularity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0100] 1-(2,5-dimethoxybenzyl)-6,7-methylenedioxy-1,2,3,4-tetrahydroisoquinoline hydrochloride SIPI 926
[0101] (1) Mix 16.3g (0.1mol) piperonylethylamine with 19.0g (0.1mol) 2.5-dimethoxyphenylacetic acid, heat at 170-180°C for 4 hours, cool, dissolve in chloroform, and then use 2NHCl, 2NNaOH Wash with water, dry over anhydrous magnesium sulfate, evaporate the solvent under reduced pressure, and the residue is washed with EtOH-H 2 O was recrystallized to obtain 22.1 g of N-(3,4-methylenedioxyphenethyl)-3,4-dimethoxyphenylacetamide with a yield of 65% and a melting point of 119-120°C.
[0102] Elemental Analysis C 19 h 21 NO 5 Calculated %: C 66.74, H 5.87, N 4.07
[0103] Found %: C 66.46, H 6.16, N 4.08
[0104] (2) Dissolve 17g (0.05mol) of the above amide in dry chloroform, add 26ml of phosphorus oxychloride, reflux for 4 hours, evaporate the solvent and excess phosphorus oxychloride under reduced pressure, and wash the residue with petroleu...
Embodiment 2
[0111] 1-(4-methoxybenzyl)-6-methoxy-7-benzyloxy-1,2,3,4-tetrahydroisoquinoline hydrochloride SIPI 1124
[0112] (1) Use 3-methoxy-4-benzyloxyphenethylamine and 4-methoxyphenylacetic acid as raw materials to prepare the corresponding amides according to Example 1 (1).
[0113] (2) take above-mentioned amide as raw material and make corresponding dihydroisoquinoline hydrochloride by the operation of embodiment 1 (2)
[0114] (3) Using the above-mentioned dihydroisoquinoline hydrochloride as a raw material, SIPI 1124 was prepared according to Example 1 (3), with a melting point of 176-177°C.
[0115] Elemental Analysis C 25 h 28 NO 3 Cl H 2 O Calculated %: C 67.63, H6.81, N3.16, C17.99
[0116] Found %: C 67.93, H7.00, N2.85, C18.19
Embodiment 3
[0118] 1-(3-Methanesulfonamidobenzyl)-6,7-methylenedioxy-1,2,3,4-tetrahydroisoquinoline hydrochloride C-391
[0119] (1) 8.5g (0.047mol) 3-nitrophenylacetic acid, 16ml SOCl 2 , mixed with 16ml benzene, heated to reflux for 4h, evaporated the solvent and excess SOCl under reduced pressure 2 In 3-nitrophenylacetyl chloride.
[0120] Dissolve 8g (0.04mol) of piperonylethylamine in 60ml of dichloroethane, and add 10% NaOH solution and 7.8g (0.039mol) of 3-nitrophenylacetyl chloride dropwise at 0-5°C. Alkanes solution, control pH8-9, add, stir at room temperature for 4h, precipitate solid, filter, wash with 5% HCl, wash with water, dry, recrystallize from ethanol to obtain 12.5g of N-(3,4-methylenedioxy Phenylethyl)-3-nitrophenylacetamide, the yield is 86%, and the melting point is 128-130°C.
[0121] (2) 10g iron powder and 5% NH 4 Mix 50ml of Cl solution, stir at 100°C for 20 minutes, then cool to 60°C, add 10g (0.03mol) N-(3,4-methylenedioxyphenethyl)-3-nitrophenylacetamide ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com