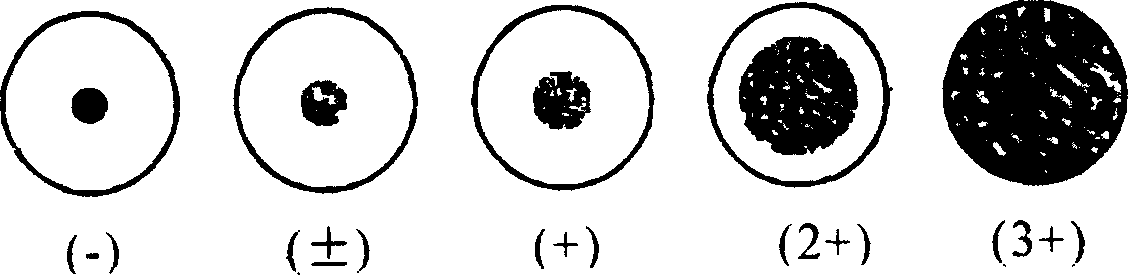
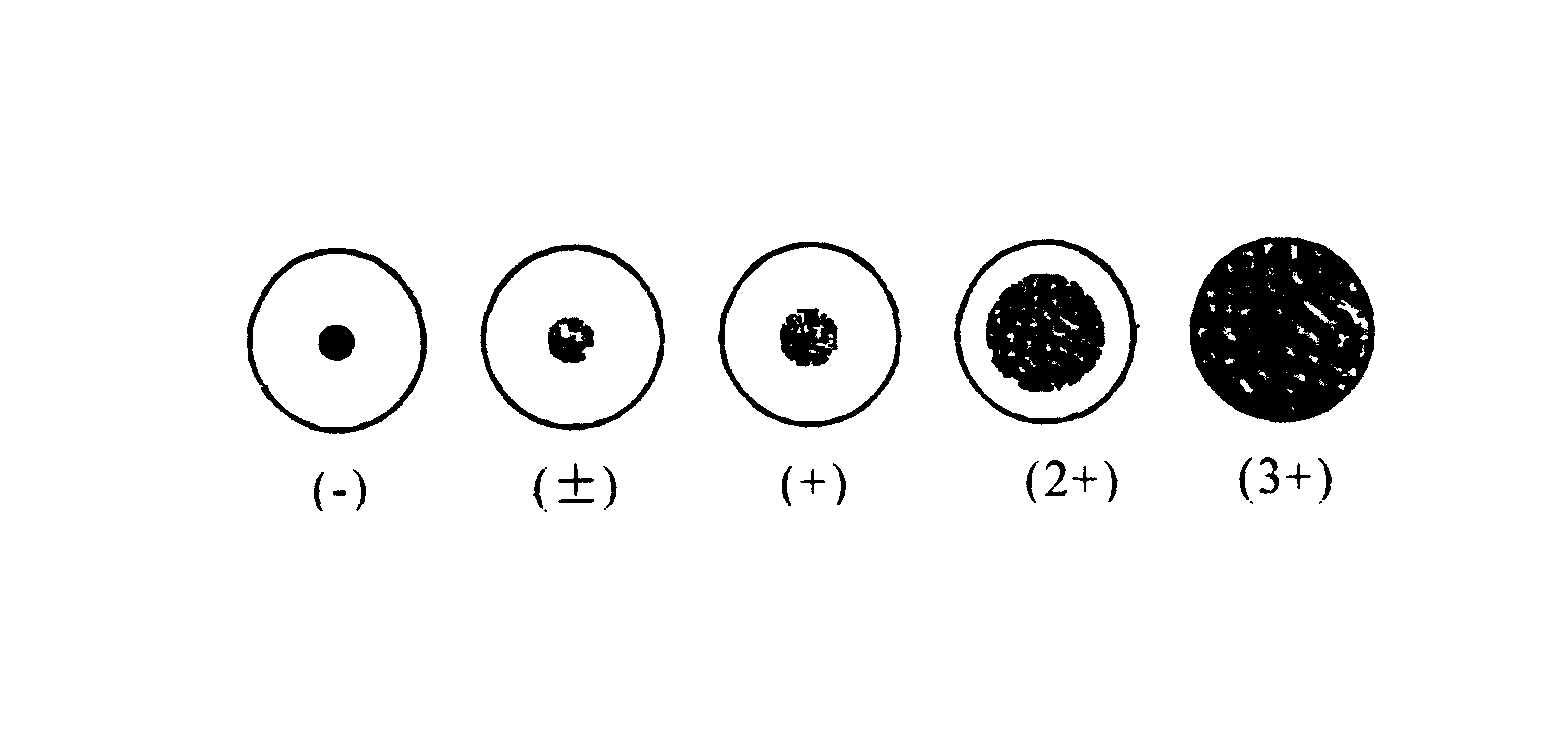Preparing and assaying method of staphylococcus aureus filtrate preparation
A staphylococcus and staphylococcus intestinal technology, applied in the field of manufacture and verification of Staphylococcus aureus filtrate preparations, can solve the problems of large quality differences, local swelling and pain, fever, antagonistic reactions, etc., to achieve high sensitivity and reduce adverse reactions Reaction, increase the effect of the purification process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Method for manufacturing staphylococcus aureus filtrate preparation
[0037] According to the regulations of China's biological products regulations, the culture medium is produced: take 5kg of fresh pig heart, remove blood vessels and fascia, wash and drain with water for injection, weigh 3kg and add about 1.5 times of water for injection, heat at 90-95°C, Keep warm for 1 hour, filter with hot gauze, add 1 times the amount of water for injection to the residue, keep warm at 90-95°C for half an hour, filter to remove the residue, combine the two filtrates, add 50g of sodium chloride and 100g of peptone, adjust the pH with dilute hydrochloric acid value to 3.0-4.0, put it at 2-8°C for 12 hours, filter it with a sterilized filter cartridge, add 2% activated carbon to the filtrate, heat and boil for 15 minutes, filter it with a sterilized filter cartridge, wait for the filtrate to cool to room temperature, and filter it with 2% activated carbon Adjust the pH value of the s...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com